Arch Hepat Res
Serum YKL-40 (Chitinase-3-Like Protein 1) Compared to APRI and FIB-4 in Predicting Liver Fibrosis in Children with Chronic Hepatitis C
Mostafa Mohamed Sira1*, Hanaa Ahmed El-Araby1, Enas Mohamed Ghoneim2, Hatem Abdel-Sattar Konsowa1, Eman Hosny El-Mwafy1 and Ibrahim A Elhenawy1
2Department of Microbiology and Immunology, National Liver Institute, Menofiya University, Menofiya, Egypt
Cite this as
Sira MM, El-Araby HA, Ghoneim EM, Konsowa HA, El-Mwafy EH, et al. (2016) Serum YKL-40 (Chitinase-3-Like Protein 1) Compared to APRI and FIB-4 in Predicting Liver Fibrosis in Children with Chronic Hepatitis C. Arch Hepat Res 2(1): 015-020. DOI: 10.17352/ahr.000006Background: Liver fibrosis is a critical factor for the treatment policy and its outcome in chronic hepatitis C virus (HCV) infection. Although liver biopsy represents the gold standard for evaluating fibrosis, it remains an invasive procedure with inherent risks. Thus, it cannot be performed frequently to monitor therapeutic outcomes specially in the pediatric population. For that, developing a non-invasive test that can predict liver fibrosis represents a growing medical need.
Objectives: to investigate serum levels of YKL-40 and their relation to liver fibrosis in children with chronic HCV.
Methods: Serum YKL-40 was measured by enzyme linked immunosorbent assay in 40 treatment-naive children with proved chronic HCV and the levels were compared according to different laboratory and histopathological parameters. Liver histopathological changes were assessed using Ishak score and compared with aspartate transaminase-to-platelet ratio (APRI) and fibrosis-4 (FIB-4) indices as simple non-invasive markers of fibrosis. YKL-40 was measured in a group of 27 age- and sex-matched healthy controls.
Results: YKL-40 was significantly higher in patients than in controls (23.79±11.59 ng/ml vs. 17.67±5.49 ng/ml; P = 0.038). YKL-40 (r = 0.36, P = 0.022), but not APRI (r = -0.176, P = 0.328) or FIB-4 (r = -0.202, P = 0.259), had a significant direct correlation with fibrosis stage. YKL-40 at a cutoff level of ≥24.82 ng/ml could discriminate patients with significant fibrosis (≥F3 Ishak) with 100% sensitivity and 80% specificity.
Conclusion: YKL-40 significantly correlated with liver fibrosis and could discriminate those with significant fibrosis with acceptable performance.
Abbreviations
Ab: Antibody; ALT: Alanine Transaminase; APRI: Aspartate Transaminase-To-Platelet Ratio; AST: Aspartate Transaminase; AUROC: Area Under ROC; ECM: Extracellular Matrix; ELISA: Enzyme-Linked Immunosorbent Assay; FIB-4: Fibrosis-4; HA: Hyaluronic Acid; HBV: Hepatitis B Virus; HCV: Hepatitis C Virus; Ig: Immunoglobulin; NPV: Negative Predictive Value; PCR: Polymerase Chain Reaction; PPV: Positive Predictive Value; ROC: Receiver Operating Characterstic
Introduction
Hepatitis C virus (HCV) infection is a serious health problem. It is estimated that over 200 million people are infected worldwide, among whom 80% develop a chronic form [1]. In children younger than 11 years, worldwide seroprevalence of HCV is 0.2% and in those older than 11 years it is 0.4% [2]. In Egyptian children, the prevalence is relatively high ranging from 3% in Upper Egypt to 9% in Lower Egypt [3]. HCV infection causes intrahepatic lobular inflammation resulting in fibrosis and eventually cirrhosis which is the cause of both hepatocellular carcinoma and cirrhosis-related hepatic decompensation [4].
Fibrosis prediction is an essential part of the assessment and management of patients with chronic liver disease. Although liver biopsy represents the gold standard for evaluating liver fibrosis and necroinflammation, it remains an invasive procedure with inherent risks. Thus, it cannot be performed repeatedly to monitor therapeutic outcomes [5,6]. Moreover, in children, biopsy is still perceived to carry a higher risk of complications, so it is less accepted than in adults. Therefore, developing a non-invasive test that can predict initial disease stage and progression over time represents a high priority and a growing medical need [7, 8]. Blood-based biomarkers offer a number of advantages over the traditional standard of fibrosis assessment of liver biopsy, including safety, cost-savings and wide spread accessibility [9].
YKL-40, also named chitinase 3–like protein 1 or human cartilage glycoprotein-39, is secreted by chondrocytes, synovial cells, and macrophages [10]. YKL refers to the first three N-terminal amino acids tyrosine (Y), lysine (K) and leucine (L) and 40 denotes its molecular mass in kilodaltons [11]. This secreted protein is not synthesized in young healthy cartilage, but is produced in cartilage from patients with osteoarthritis [12].
Chitinase-like proteins are a family of mediators increasingly associated with infection, T cell-mediated inflammation, wound healing, allergy and asthma [13]. The physiological function of YKL-40 is not known, but the pattern of its expression in normal and diseased states suggests that it could function in remodelling of the extracellular matrix (ECM) or in tissue inflammation. YKL-40 was found to be high in patients with hepatic fibrosis [11].
We aimed to investigate serum levels of YKL-40 and their relation to liver fibrosis in children with chronic HCV infection.
Materials and Methods
Study population
This prospective study included 40 children with proved chronic HCV infection recruited from the outpatient and inpatient of Pediatric Hepatology, Gastroenterology and Nutrition department, National Liver Institute, Menofiya University. Diagnosis of chronic hepatitis C was based on the presence of persistently positive HCV-RNA as detected by polymerase chain reaction (PCR) for more than 6 months [14,15], supported by the histopathological feature of HCV infection in liver biopsy. Criteria for inclusion were children aged up to 18 years with compensated chronic HCV infection, who have no other associated liver disease [autoimmune hepatitis, Wilson disease, alpha-1 antitrypsin deficiency, hepatitis B virus (HBV) infection]. Liver biopsy was mandatory for enrollment. Patients with decompensated cirrhosis, any other cause of liver disease associating HCV infection or immunologically mediated diseases, were excluded from the study. A second group of 27 healthy children with no signs or symptoms of liver disease or any other diseases, normal liver transaminases and negative anti-HCV antibody (Ab), served as controls. A signed informed consent was obtained from the legal gaurdians of all the patients and controls before enrollment in the study. The study was approved by the Research Ethics Committee of the National Liver Institute.
Laboratory investigations
Laboratory investigations, including liver function tests, complete blood count, kidney function tests, serum autoantibodies (anti-nuclear antibodies [Ab], anti-smooth muscle Ab, and liver-kidney microsomal Ab) and prothrombin time were performed for all the patients. Serum viral markers were performed using enzyme-linked immunosorbent assay (ELISA) according to the manufacturer instructions; HCV Ab (Innogenetics, Ghent, Belgium), HBV surface antigen, HBV core immunoglobulin (Ig)M and IgG Abs (all from Dia Sorin, Saluggia, Italy). Real-time PCR for HCV-RNA was performed using COBAS Ampliprep/COBAS TaqMan, Roche Molecular Systems, Inc., Branchburg, NJ, 08876 USA (detection limit was 15 IU/mL). Serum YKL-40 levels were assayed using ELISA kit (R&D Systems, Inc. Cat # DC3L10, Canada, USA) according to the manufacturer instructions. Serum samples of the patients were collected, maximally, within 6 months of liver biopsy [16]. All the controls were tested for aspartate transaminase (AST), alanine transaminase (ALT), complete blood count, HCV Ab and serum YKL-40.
Liver biopsy and histopathological evaluation
Liver biopsy was performed using an ultrasonography-guided TruCut needle for all the patients. Specimens were fixed in formalin, embedded in paraffin and stained with hematoxylin and eosin, Masson’s trichrome, reticulin and Perl’s stains. Hepatic necroinflammatory activity and liver fibrosis were evaluated according to Ishak staging and grading score. Necroinflammatory activity was classified into minimal (score 1-3), mild (score 4-8), moderate (score 9-12), and severe (score 13-18) [17]. Fibrosis was classified into mild (stage 1), moderate (stages 2-3), and severe fibrosis or cirrhosis (stages 4-6) [3]. Significant fibrosis was defined as Ishak score of 3 or more (presence of bridging fibrosis) [18]. AST-to-platelet ratio index (APRI) and fibrosis-4 (FIB-4) index were calculated according to the formula; APRI = AST / upper limit of normal x 100 / platelet count (109/L), FIB-4 = Age (years) x AST / platelet count (109/L) x (ALT)1/2 [19].
Statistical analysis
Descriptive results were expressed as mean ± standard deviation (SD) or number (percentage). For quantitative data, statistical significance was tested by either Mann-Whitney U non-parametric test or Kruskal-Wallis test. For qualitative data, significance between groups was tested by Chi-square test or Fisher exact test. Correlation was tested by Spearman’s test. The cutoff values for optimal clinical performance was determined from the receiver operating characterstic (ROC) curve. The diagnostic performance was measured by the area under ROC (AUROC) and presented as sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). Results were considered significant if P-value < 0.05. Statistical analysis was performed using SPSS version 13 (SPSS Inc, Chicago, IL, USA).
Results
Study population characteristics
The study included 40 children with chronic HCV infection. They were 9 females and 31 males. Their mean age was 12.68 ± 3.87 years, ranging from 4 to 18 years. A second group of 27 age- and sex-matched (P>0.05 for both) healthy children served as controls. They were 11 females and 16 males. Their mean age was 13.37 ± 2.98 years, ranging from 7 to 17 years. The majority of patients (85%) were asymptomatic while 6 (15%) children presented with abdominal enlargement. Clinicaly, 6 (15%) children had hepatomegaly, 5 (12.5%) had splenomegaly and none had jaundice or ascites. Fibrosis stage ranged from F1 to F3 and activity grade ranged from A2 to A10. The majority of patients (87%) had either F1 or F2 fibrosis and mild activity was found in 85% of patients (Table 1).
Histopathological findings in patients with normal versus elevated transaminases
All the patients had mild to moderate fibrosis and the majority had minimal to mild activity in liver biopsy while one patient only had moderate activity. Nearly half of them had normal transaminases (44.4%, 48.4%, 80% and 41.2% with mild fibrosis, moderate fibrosis, minimal activity and mild activity respectively) (Table 2).
YKL-40 according to disease severity and correlation with laboratory and histopathological parameters
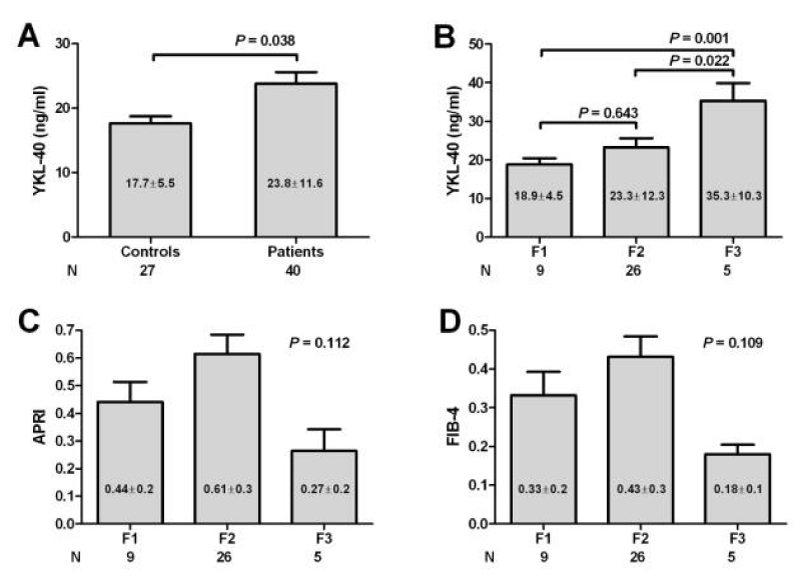
The mean value of serum YKL-40 was significantly higher in patients than in controls (23.8±11.6 ng/ml vs. 17.7±5.5 ng/ml; P = 0.038) (Fig 1 A). There was no statistical significant difference in the mean level of YKL-40 when comparing patients with different activity grades (P = 0.44), and patients with normal transaminases versus those with elevated transaminases (P = 0.303) (Table 3) but the levels tended to be higher in those with higher activity grade. In addition, there was a significant direct correlation between YKL-40 and the stage of fibrosis (P = 0.022) while there was no significant correlation with the other studied laboratory parameters, (Table 4). On the other hand, there was no correlation between APRI (r = -0.176, P = 0.328) or FIB-4 (r = -0.202, P = 0.259) with the stage of fibrosis (data not shown).
YKL-40 is associated with higher stages of liver fibrosis
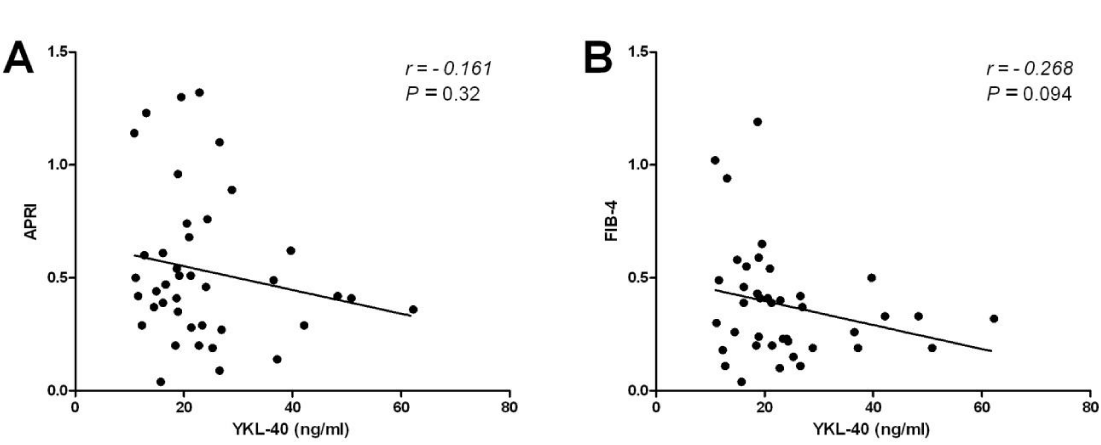
Serum YKL-40 was at its lowest (18.9 ± 4.5 ng/ml) in patients with F1, increasing in patients with F2 (23.3 ± 12.3 ng/ml) and reaching the highest level in F3 (35.3 ± 10.3 ng/ml). Though the levels in F1 and F2 had no significant difference, the levels in F3 were significantly higher than those in F1 and F2 (Figure 1A,B). On the other hand, there was no significant statistical difference among the mean values of APRI (P = 0.112) and FIB-4 (P = 0.109) according to individual fibrosis stages (Figure 1C,D). Furthermore, both APRI and Fib-4 did not show any significant correlation with YKL-40 (Figure 2).
Clinical performance of YKL40 in predicting significant fibrosis
Serum YKL-40 at a cutoff level of 24.82 ng/ml could discriminate patients with significant fibrosis (≥F3 Ishak) with 100% sensitivity, 80% specificity, 41.7% PPV, 100% NPV and AUROC of 86.9% (Figure 3).
Discussion
Whilst the presence of mild fibrosis on biopsy can be a reassuring finding, the identification of advanced fibrosis is critical to the management of patients with chronic liver disease. The development of robust tools to non-invasively assess liver fibrosis has dramatically enhanced clinical decision making in patients with chronic liver disease, allowing a rapid and informed judgment of disease stage and prognosis [20].
Selection of the appropriate non-invasive predictor of liver fibrosis represents a challenge for hepatologists. Non-invasive biomarkers of fibrosis can be classified into class I and class II. Class I biomarkers depend on the pathophysiology of fibrosis such as collagen and hyaluronic acid (HA), wherase class II biomarkers depend mainly on laboratory tests to estimate the degree of fibrosis such as APRI and FIB-4 [21].
APRI and FIB-4 have been of interest to clinicians because they are simple to calculate and readily available from hospital or clinic laboratories during the usual patient care [19]. In our study, APRI and FIB-4 showed no significant statistical difference (P > 0.05 for both) among fibrosis stages. In hand with our results, Diaz et al., [22] reported that APRI did not predict fibrosis in pediatric patients. Contrary to our results, De Ledinghen et al., [23] found that APRI was of benefit in predicting cirrhosis in children with various chronic liver diseases. The majority of reports using APRI and FIB-4 showed a significant performance in discriminating F0-F2 from F3-F4 Metavir score [19], or discriminating F0- F3 from F4-F6 Ishak score [24]. Such advanced stages of fibrosis or cirrhosis were not detected in our study population.
APRI and FIB4 reflect alterations in hepatic functions rather than in ECM metabolism. Since, several reports have described normal transaminases levels in about 25%–30% of chronic HCV patients, there may be a potential advantage in assessing serum direct fibrosis markers that do not involve transaminases [25]. In our study, 47.5% (19/40) of patients had normal transaminases despite the presence of mild or moderate fibrosis. This may explain the poor association of APRI and FIB-4 with fibrosis in our study compared to that of YKL-40. In addition, we didn’t find any significant correlation between APRI or FIB-4 with YKL-40 serum levels. This may be due to absence of higher stages of fibrosis which may demonstrate the ability of APRI and FIB-4 in predicting higher stages of fibrosis or cirrhosis as demonstrated by other studies [19,23].
YKL-40 is an emerging new inflammation biomarker. The YKL-40 gene consists of ten exons located within 8 kb of DNA on human chromosome 1q32.1, and encodes a protein of 383 amino acids. YKL-40 has a role in cell proliferation and differentiation, angiogenesis, inflammation, remodeling of the ECM, and the innate immune response [26].
In the current study, YKL-40 was significantly higher in patients than in controls and was highest in F3 when compared to F2 (P = 0.022) and F1 (P = 0.001). In hand with these results, Abo El-Asrar et al., [27] reported that YKL-40 was significantly higher among children with HCV (P = 0.004) than in healthy controls. YKL-40 correlated with the degree of hepatic fibrosis being highest among patients with F4 stage (P < 0.001). In addition, Zheng et al., [28] found that YKL-40 in adult patients with hepatic fibrosis was significantly higher when compared to healthy controls.
In patients with no or minimal fibrosis at presentation, antiviral treatment could possibly be delayed due to the mild nature of the disease and the slow progression of liver fibrosis, while in those with significant fibrosis, antiviral treatment is a priority [29]. For that, identifiying patients with significant fibrosis is of utmost importance. In the current study, YKL-40 at a cutoff level of 24.82 ng/ml could discriminate those with significant fibrosis with 100% sensitivity and 80% specificty. Mehta et al., [30] evaluated serum YKL-40 in various categories of fibrosis due to HCV infection where it could predicted advanced fibrosis and cirrhosis.
Saitou et al., [31] studied both YKL-40 and HA in predicting HCV-associated liver fibrosis. YKL-40 was superior to HA for predicting severe fibrosis (F2-F4) from mild fibrosis (F0-F1) (YKL-40, AUC = 0.809; HA, AUC = 0.805). After interferon therapy, only YKL-40 values significantly decreased not only in the responder group, but also in the non-responder group (P = 0.03). These findings mean that YKL-40 may be a useful non-invasive marker to estimate the degree of liver fibrosis but is probably not a good tool to evaluate the efficacy of interferon therapy in such patients.
YKL-40 was used for evaluation of liver fibrosis due to other etiologies as HBV. Toson et al., [32] found a significant increase of YKL-40 with increasing the stage of fibrosis in HBV infection. It also dramatically enhances the diagnostic power of other non-invasive scores in discriminating the stages of the disease. Contrarily, Lebenztjn et al., [33] and Lee et al., [34] found that the ability of serum YKL-40 to differentiate children with advanced liver fibrosis from those with mild fibrosis was not significant. Our results showed no significant difference of YKL-40 among different activity grades (P = 0.44). Similar to our results, Lebenztjn et al., [33] reported that YKL-40 is not a good predictor of histological inflammation either. Yet, in the current study, there was a trend of increased YKL-40 with increasing activity grade.
We have previously studied other serological non-invasive markers such as serum complement C4a [35] and serum inter-alpha-trypsin inhibitor heavy chain 4 [36] in children with chronic HCV. It can be concluded that none of these markers alone can reach an accurate prediction of liver fibrosis. Compared to the current study, YKL-40 appears better. For that, a combination of different markers can enhance the performance in predicting fibrosis more than individual markers do.
There has been a growing interest in the molecular basis of YKL-40 in liver fibrosis. Berres et al., [37] reported that YKL-40 promoter C allele determines YKL-40 serum levels and is associated with the severity of HCV-induced liver fibrosis. While homozygous carriers of the G allele were protected from severe fibrosis. These results suggest a functional role of YKL-40 in liver fibrogenesis that make it a candidate to be a non-invasive marker of fibrosis. In addition, Sarma et al., [38] found that micro RNA (miRNA)- 449a has a role in modulating the expression of YKL-40 after HCV infection. In fibroblasts and synovial cells, YKL-40 mediates a mitogenic effect through initiation of mitogen activated protein kinase and phosphoinositide 3-kinase pathways. It is therefore of major importance to explore if YKL-40 could become a target for the development of new fibrosis therapeutics [26].
In conclusion, our study demonstrated that YKL-40 serum levels were associated with higher stages of liver fibrosis and significantly discriminate those with significant fibrosis with acceptable performance. Future studies on YKL-40 as a possible therapeutic target in liver fibrosis is worthy.
Funding source
This study was funded by National Liver Institute, Menofiya University, Egypt, without any particular role in the study design, data collection and analysis or the writing of the report.
- Valva P, Casciato P, Lezama C, Galoppo M, Gadano A, et al. (2013) Serum apoptosis markers related to liver damage in chronic hepatitis C: sFas as a marker of advanced fibrosis in children and adults while M30 of severe steatosis only in children. PLoS One 8: e53519. Link: https://goo.gl/xpG7DM
- Yazigi N, Balistreri WF (2011) Viral hepatitis. In Nelson Textbook of pediatrics (Kliegman RM, Behrman RE, Jenson HB, Stanton BF, Eds.) 19 ed. 1393-1400.
- El-Raziky MS, El-Hawary M, Esmat G, Abouzied AM, El-Koofy N, et al. (2007) Prevalence and risk factors of asymptomatic hepatitis C virus infection in Egyptian children. World J Gastroenterol 13: 1828-1832. Link: https://goo.gl/EnTJR4
- Shackel NA, McGuinness PH, Abbott CA, Gorrell MD, McCaughan GW (2003) Novel differential gene expression in human cirrhosis detected by suppression subtractive hybridization. Hepatology 38: 577-588. Link: https://goo.gl/112sfF
- Bravo AA, Sheth SG, Chopra S (2001) Liver biopsy. N Engl J Med 344: 495-500. Link: https://goo.gl/25JbQl
- Thampanitchawong P, Piratvisuth T (1999) Liver biopsy: complications and risk factors. World J Gastroenterol 5: 301-304. Link: https://goo.gl/HpSysh
- Afdhal NH, Nunes D (2004) Evaluation of liver fibrosis: a concise review. Am J Gastroenterol 99: 1160-1174. Link: https://goo.gl/d4t2bI
- Martinez SM, Crespo G, Navasa M, Forns X (2011) Noninvasive assessment of liver fibrosis. Hepatology 53: 325-335. Link: https://goo.gl/wCB2sD
- Adams LA (2011) Biomarkers of liver fibrosis. J Gastroenterol Hepatol 26: 802-809. Link: https://goo.gl/pEu0eB
- Johansen JS, Jensen BV, Roslind A, Nielsen D, Price PA (2006) Serum YKL-40, a new prognostic biomarker in cancer patients? Cancer Epidemiol Biomarkers Prev 15: 194-202. Link: https://goo.gl/t8SXuU
- Johansen JS, Christoffersen P, Moller S, Price PA, Henriksen JH, et al. (2000) Serum YKL-40 is increased in patients with hepatic fibrosis. J Hepatol 32: 911-920. Link: https://goo.gl/cwLcza
- Recklies AD, Ling H, White C, Bernier SM (2005) Inflammatory cytokines induce production of CHI3L1 by articular chondrocytes. J Biol Chem 280: 41213-41221. Link: https://goo.gl/YQHZon
- Sutherland TE, Maizels RM, Allen JE (2009) Chitinases and chitinase-like proteins: potential therapeutic targets for the treatment of T-helper type 2 allergies. Clin Exp Allergy 39: 943-955. Link: https://goo.gl/6u8ZRy
- Alisi A, Comparcola D, Nobili V (2010) Treatment of chronic hepatitis C in children: is it necessary and, if so, in whom? J Hepatol 52: 472-474. Link: https://goo.gl/sZwvek
- Chen SL, and Morgan TR (2006) The natural history of hepatitis C virus (HCV) infection. Int J Med Sci 3: 47-52. Link: https://goo.gl/u8NwsZ
- Brown KS, Keogh MJ, Tagiuri N, Grainge MJ, Presanis JS, et al. (2007) Severe fibrosis in hepatitis C virus-infected patients is associated with increased activity of the mannan-binding lectin (MBL)/MBL-associated serine protease 1 (MASP-1) complex. Clin Exp Immunol 147: 90-98. Link: https://goo.gl/zlAo1P
- Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, et al. (1995) Histological grading and staging of chronic hepatitis. J Hepatol 22: 696-699. Link: https://goo.gl/IHZq81
- Wai C-T, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, et al. (2003) A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 38: 518-526. Link: https://goo.gl/TcdwFT
- Holmberg SD, Lu M, Rupp LB, Lamerato LE, Moorman AC, et al. (2013) Noninvasive serum fibrosis markers for screening and staging chronic hepatitis C virus patients in a large US cohort. Clin Infect Dis 57: 240-246. Link: https://goo.gl/NeHMOl
- Chin JL, Pavlides M, Moolla A, Ryan JD (2016) Non-invasive Markers of Liver Fibrosis: Adjuncts or Alternatives to Liver Biopsy? Front Pharmacol 7: 159. Link: https://goo.gl/DJO04T
- Gressner AM, Gao C-F, Gressner OA (2009) Non-invasive biomarkers for monitoring the fibrogenic process in liver: a short survey. World J of Gastroenterol 15: 2433-2440. Link: https://goo.gl/sX1llH
- Diaz JJ, Gura KM, Roda J, Perez-Atayde AR, Duggan C, et al. (2013) Aspartate aminotransferase to platelet ratio index correlates with hepatic cirrhosis but not with fibrosis in pediatric patients with intestinal failure. J Pediatr Gastroenterol Nutr 57: 367-371. Link: https://goo.gl/qpgBoF
- de Lédinghen V, Le Bail B, Rebouissoux L, Fournier C, Foucher J, et al. (2007) Liver Stiffness Measurement in Children Using FibroScan: Feasibility Study and Comparison With Fibrotest, Aspartate Transaminase to Platelets Ratio Index, and Liver Biopsy. J Pediatr Gastroenterol Nutr 45: 443-450. Link: https://goo.gl/QW5UoN
- Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, et al. (2006) Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection, Hepatology 43: 1317-1325. Link: https://goo.gl/CllbW0
- Valva P, Casciato P, Diaz Carrasco JM, Gadano A, Galdame O, et al. (2011) The role of serum biomarkers in predicting fibrosis progression in pediatric and adult hepatitis C virus chronic infection. PLoS One 6: e23218. Link: https://goo.gl/96hJql
- Tao H, Yang JJ, Shi KH, Huang C, Zhang L, et al. (2014) The significance of YKL-40 protein in liver fibrosis. Inflamm Res 63: 249-254. Link: https://goo.gl/upSDth
- Abo-El-Asrar M, Elbarbary NS, Ismail EAR, Elshenity AM (2016) Serum YKL-40 in young patients with β-thalassemia major: Relation to hepatitis C virus infection, liver stiffness by transient elastography and cardiovascular complications. Blood Cells Mol Dis 56: 1-8. Link: https://goo.gl/adJMX7
- Zheng M, Wei-Min C, Jun-Kang Z, Shao-Ming Z, Rong-Hua L (2005) Determination of serum levels of YKL-40 and hyaluronic acid in patients with hepatic fibrosis due to schistosomiasis japonica and appraisal of their clinical value. Acta tropica 96: 148-152. Link: https://goo.gl/vms7LS
- Yano M, Kumada H, Kage M, Ikeda K, Shimamatsu K, et al. (1996) The long-term pathological evolution of chronic hepatitis C. Hepatology 23: 1334-1340. Link: https://goo.gl/nY7rHB
- Mehta P, Ploutz-Snyder R, Nandi J, Rawlins SR, Sanderson SO, et al. (2008) Diagnostic Accuracy of Serum Hyaluronic Acid, FIBROSpect II, and YKL-40 for Discriminating Fibrosis Stages in Chronic Hepatitis C. Am J Gastroenterol 10: 928-936. Link: https://goo.gl/qrj2kj
- Saitou Y, Shiraki K, Yamanaka Y, Yamaguchi Y, Kawakita T, et al. (2005) Noninvasive estimation of liver fibrosis and response to interferon therapy by a serum fibrogenesis marker, YKL-40, in patients with HCV-associated liver disease. World J Gastroenterol 11: 476-481. Link: https://goo.gl/QQwDZR
- Toson EA, Shiha GE, EL-Saied EH, Samir W, ELbasiony M, et al. (2016) Can YKL-40 improve the diagnostic power of non-invasive fibrogenic staging in chronic hepatitis B virus infected patients? EJPMR 11: 70-78. Link: https://goo.gl/Zlgbeh
- Lebensztejn D, Skiba E, Werpachowska I, Sobaniec-Lotowska M, Kaczmarski M (2007) Serum level of YKL-40 does not predict advanced liver fibrosis in children with chronic hepatitis B. Adv Med Sci 52: 120-124. Link: https://goo.gl/UkaoGC
- Lee CK, Perez-Atayde AR, Mitchell PD, Raza R, Afdhal NH, et al. (2013) Serum Biomarkers and Transient Elastography as Predictors of Advanced Liver Fibrosis in a United States Cohort: The Boston Children's Hospital Experience. J Pediatr 163: 1058-1064. Link: https://goo.gl/VNFamJ
- Behairy BE, El-Mashad GM, Abd-Elghany RS, Ghoneim EM, Sira MM (2013) Serum complement C4a and its relation to liver fibrosis in children with chronic hepatitis C. World J Hepatol 5: 445-451. Link: https://goo.gl/GGvzhX
- Sira MM, Behairy BE, Abd-Elaziz AM, Abd Elnaby SA, Eltahan EE (2014) Serum Inter-Alpha-Trypsin Inhibitor Heavy Chain 4 (ITIH4) in Children with Chronic Hepatitis C: Relation to Liver Fibrosis and Viremia. Hepa Res Treat 2014: 307942: 7 Link: https://goo.gl/rRCyOA
- Berres ML, Papen S, Pauels K, Schmitz P, Zaldivar MM, et al. (2009) A functional variation in CHI3L1 is associated with severity of liver fibrosis and YKL-40 serum levels in chronic hepatitis C infection. J Hepatol 50: 370-376. Link: https://goo.gl/DWPpJc
- Sarma NJ, Tiriveedhi V, Subramanian V, Shenoy S, Crippin JS, et al. (2012) Hepatitis C virus mediated changes in miRNA-449a modulates inflammatory biomarker YKL40 through components of the NOTCH signaling pathway. PLoS One 7: e50826. Link: https://goo.gl/v3ORwN
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
