Archives of Hematology Case Reports and Reviews
Hemophagocytic lymphohistiocytosis secondary to epstein-barr virus reactivation in a patient with COVID-19
Nurfiza Ladak1*, Kenneth Csehak2, Justin Chan3 and Farnoush Moen4
2Hematology & Medical Oncology Fellow, NYU Grossman School of Medicine, USA
3Assistant Professor, Department of Medicine, NYU Grossman School of Medicine Director, Infection Prevention and Control, Bellevue Hospital Center, USA
4Clinical Assistant Professor, Department of Pathology, NYU Grossman School of Medicine Director, Hematology and Hematopathology, Bellevue Hospital Center, USA
Cite this as
Ladak N, Csehak K, Chan J, Moen F (2022) Hemophagocytic lymphohistiocytosis secondary to epstein-barr virus reactivation in a patient with COVID-19. Arch Hematol Case Rep Rev 7(1): 006-008. DOI: 10.17352/ahcrr.000036Copyright License
© 2022 Ladak N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Hemophagocytic lymphohistiocytosis (HLH) is a recognized complication of severe coronavirus disease 2019 (COVID-19). However, this phenomenon has been reported most often in the setting of acute infection. Here we present a case of a patient with a history of COVID-19 that subsequently developed HLH weeks after treatment and discharge from the hospital. Upon re-admission, subsequent work-up demonstrated the patient was experiencing Epstein-Barr virus (EBV) reactivation. As EBV infection is a known etiological trigger of HLH, this case provides an alternative mechanism for HLH seen in patients with a history of COVID-19 who present after the resolution of acute symptomatology.
Introduction
Infection with severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) is associated with a complex host immune response, with the loss of immune regulation to the point of exacerbation ultimately implicated in disease progression. Such a hyperinflammatory clinical picture in a subgroup of patients develops into hemophagocytic lymphohistiocytosis (HLH), a recognized complication in patients with coronavirus disease 2019 (COVID-19) [1,2]. In addition, reports of reactivation of opportunistic infections, such as Epstein-Barr Virus (EBV) in the setting of COVID-19 infection have also been described [3,4]. Here we present a unique case of EBV reactivation in a patient with COVID-19 infection and subsequent HLH.
Case report
An 84-year-old Hispanic male with a medical history of dementia, hypertension, cerebrovascular accident and recent COVID-19 infection presented to the emergency department with altered mental status, urinary incontinence, and decreased oral intake.
Of note, he was hospitalized one month prior with COVID-19 that was complicated by prolonged hypoxia and shortness of breath, requiring 3-4 liters of oxygen by nasal cannula. He received dexamethasone and remdesivir and was admitted to the medicine service for four days.
Upon improvement, he was discharged home on supplemental oxygen to be used on exertion. On subsequent follow-up in the geriatric clinic post-discharge, he was noted to be tachycardic with a low-grade fever. He also had ongoing confusion, fatigue, and dyspnea on exertion. He was therefore sent to the emergency department for further workup. On presentation at the emergency department, he was afebrile at 98.4°F. Yet he remained tachycardic at 125bpm, with a respiratory rate of 24 and a SpO2 of 95% His CRP was elevated at 227mg/L with a ferritin of 812.8 ng/mL. Hemoglobin was 10.3g/dL, white blood cell count 7.17×103/mcL and platelets 255×103/mcL. Given his history and persistent symptoms, it was suspected that the patient could be having a post-COVID inflammatory syndrome, or could have a superimposed bacterial infection. He was admitted for further workup.
A full workup including lumbar puncture was unremarkable. His chest Computed Tomography Angiography (CTA) was negative for a pulmonary embolism and brain Magnetic Resonance Imaging (MRI) displayed no acute changes, with evidence of his prior lacunar infarct.
However, his inflammatory markers continued to climb and his fever persisted despite the use of broad-spectrum antibiotics. Peripheral blood flow cytometry revealed the persistence of a known Chronic Lymphocytic Lymphoma (CLL) clone, with the extent of involvement stable at approximately 5%.
Infectious disease consult determined that the patient had a high EBV viral load, reaching up to 25,200IU/mL over the course of his admission. A finding that was suspicious for EBV reactivation, offering a likely etiology for his fevers. Furthermore, it was noted that his hemoglobin continued to trend down, as did his platelets and white count. Given the patient’s febrile illness associated with marked inflammation with a CRP peaking at 279.19mg/L and ferritin peaking at 11,000ng/mL, in the setting of EBV viremia and concomitant pancytopenia, the concern for HLH secondary to EBV was raised and a bone marrow biopsy was obtained.
His subsequent extended hospital course was complicated by worsening pancytopenia, with his hemoglobin reaching a nadir of 6.3g/dL requiring pRBC transfusions, his platelets decreasing to 20,000/mcL, and white blood cell count with an absolute neutrophil count as low as 780/mcL. He also experienced metabolic derangement in the form of hypernatremia and the development of a small bowel obstruction. He was evaluated by surgery who recommended no surgical intervention given the severity of his illness. The medical team, family, and palliative care discussed the patient and ultimately the family decided to proceed with comfort care.
Unfortunately, the patient continued to deteriorate and death was pronounced with family at the bedside, approximately 6 weeks post-admission.
Histopathological findings
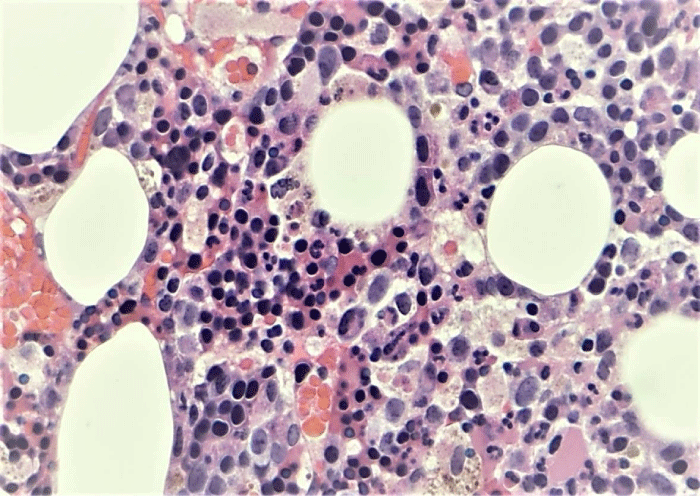
Bone marrow biopsy revealed trabecular bone and marrow elements. The marrow was hypercellular for age, with cellularity estimated between 30-70%. There was an increase in erythroid lineage noted, displaying full maturation, as well as an increase in megakaryocytes with occasional dyspoiesis including small hypo-lobated forms. Myeloid maturation was present and complete (Figure 1). Iron stores were markedly increased and reticulin fibrosis was increased at 3/3.
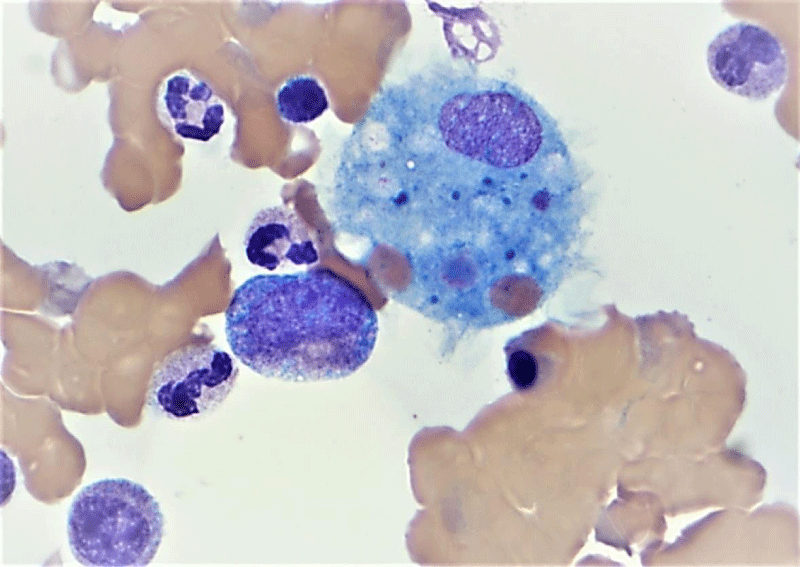
Most notably, a patchy infiltration of histiocytes containing iron granules and erythroid elements was also present. Hemophagocytosis was also appreciated on the aspirate smear and the iron stain of the aspirate smear (Figure 2).
Disscusion
Here we present a case of HLH secondary to EBV reactivation in the setting of a recent COVID- 19 infection. HLH is an aggressive and life-threatening syndrome of excessive immune activation. HLH may be inherited (primary) or may be secondary to malignancy, systemic autoimmunity, or viral infection. The clinical distinction between the two may be ambiguous in cases as in many patients with an underlying genetic defect an infectious trigger is still also present [5]. Viral infections are the most common cause of secondary HLH, with viruses of the herpes family, especially EBV being the most frequent. EBV-associated HLH is associated with EBV infection of T or NK cells rather than B cells [6].
Our understanding of HLH has evolved and the current consensus is that HLH is a syndrome of a dysregulated inflammatory response, resulting in tissue destruction and continued abnormal activation of the immune system [7,8]. This hyperinflammatory state is thought to be a result of the absence of normal feedback deactivation of macrophages. Natural Killer (NK) cells and cytotoxic T-lymphocytes, which typically act to eliminate activated macrophages, fail to do so in HLH. This results in a loss of feedback regulation that typically dampens the immune response. Persistent activation of macrophages and cytotoxic T-lymphocytes leads to excessive cytokine production [8]. These cytokines are thought to be responsible for the multiorgan failure and the high mortality of this syndrome (“cytokine storm”). In addition to the antigen presentation and cytokine production by macrophages described above, hemophagocytosis is also seen [7,9]. This occurs when host red blood cells, platelets, or white blood cells are taken up by histiocytes or macrophages and are seen in the cytoplasm. This phenomenon can be observed in tissue or bone marrow biopsies and is supportive of a diagnosis of HLH.
Clinically, a diagnosis of HLH can be made if 5 of the following 8 diagnostic criteria are met: fever (peak temperature of >38.5° C for >7 days), splenomegaly (spleen palpable >3cm below costal margin), cytopenia involving >2 cell lines, hypertriglyceridemia or hypofibrinogenemia, hemophagocytosis (in biopsy samples of bone marrow, spleen, or lymph nodes), low or absent NK cell activity, serum ferritin > 500ng/mL and elevated soluble interleukin-2 (CD25) levels [10]. It is recommended that treatment is commenced as soon as a diagnosis of HLH is considered, as delays in therapy pertain to a worse prognosis [11]. The goal of therapy is to suppress the uncontrolled inflammatory cascade that occurs in HLH. This is most often accomplished through induction therapy that consists of weekly dexamethasone and etoposide [11]. If a patient fails to improve, they are continued on therapy with the ultimate goal of allogenic stem cell transplant. What is unique in this case is the patient’s recent history of COVID-19 infection and subsequent rising EBV titers consistent with EBV reactivation. EBV reactivation in COVID-19 has been described previously. Paolucci, et al. [3]. Examined opportunistic viral DNA increase in Italian patients with COVID-19. They found a correlation between reduced CD8+ T cells and NK counts, EBV DNA levels, and COVID-19 severity. Other opportunistic viral infections were not observed. In addition, they found that the median EBV DNA levels in patients with severe COVID-19 were significantly higher than in patients with mild COVID-19 disease, supporting the conclusion that patients with severe infection had greater immune impairment. These findings are consistent with the findings of Chen, et al. [4] who found a high incidence of co-infection with EBV/ SARS-CoV-2. Co-infection was associated with fever and higher C-reactive protein (CRP) and Aspartate Aminotransferase (AST) values compared to infection with SARS-CoV-2 alone. They also conclude that EBV reactivation may be associated with the severity of COVID-19.
Conclusion
COVID-19 infection results in a complex interplay of immune responses which we are only just beginning to understand. Although HLH has been documented and is an accepted complication of immune over-activation in COVID-19 [1,2], an alternative etiology of EBV reactivation in the setting of COVID-19 and subsequent HLH also exists. This mechanism may underlie HLH that presents in the setting of sub-acute infection or “long COVID”/post-acute sequelae, seen in our case.
- Allen J, McCambridge MM, Kincaid H, Kalter JA (2021) Incidence of Secondary Hemophagocytic Lymphohistiocytosis in Critically-Ill COVID-19 Patients. Cureus 13: e16735. Link: https://bit.ly/3gMl6jh
- Schnaubelt S, Tihanyi D, Strassl R, Schmidt R, Anders S, et al. (2021) Hemophagocytic lymphohistiocytosis in COVID-19: Case reports of a stepwise approach. Medicine (Baltimore) 100: e25170. Link: https://bit.ly/3I6Q7ut
- Paolucci S, Cassaniti I, Novazzi F, Fiorina L, Piralla A, et al. (2021) San Matteo Pavia COVID-19 Task Force. EBV DNA increase in COVID-19 patients with impaired lymphocyte subpopulation count. Int J Infect Dis 104: 315-319. Link: https://bit.ly/36f8ejH
- Chen T, Song J, Liu H, Zheng H, Chen C (2021) Positive Epstein–Barr virus detection in coronavirus disease 2019 (COVID-19) patients. Sci Rep 11: 10902. Link: https://bit.ly/3LsLxsi
- Kleynberg RL, Schiller GJ (2012) Secondary hemophagocytic lymphohistiocytosis in adults: an update on diagnosis and therapy. Clin Adv Hematol Oncol 10: 726-732. Link: https://bit.ly/3oOCsQK
- Kimura H, Fujiwara S (2019) Overview of EBV-Associated T/NK-Cell Lymphoproliferative Diseases. Front Pediatr 6: 417. Link: https://bit.ly/3sJXZva
- Gupta S, Weitzman S (2010) Primary and secondary hemophagocytic lymphohistiocytosis: clinical features, pathogenesis and therapy. Expert Rev Clin Immunol 6: 137-154. Link: https://bit.ly/3pblg8D
- Dalal BI, Vakil AP, Khare NS, Wang SY, Richards MJ, et al. (2015) Abnormalities of the lymphocyte subsets and their immunophenotype, and their prognostic significance in adult patients with hemophagocytic lymphohistiocytosis. Ann Hematol 94: 1111-1117. Link: https://bit.ly/3rRHkXz
- Risma K, Jordan MB (2012) Hemophagocytic lymphohistiocytosis: updates and evolving concepts. Curr Opin Pediatr 24: 9-15. Link: https://bit.ly/3HUrD7i
- Rosado FG, Kim AS (2013) Hemophagocytic Lymphohistiocytosis: An Update on Diagnosis and Pathogenesis. Am J Clin Pathol 139: 713-727. Link: https://bit.ly/3gR9mfa
- Janka GE, Lehmberg K (2013) Hemophagocytic lymphohistiocytosis: pathogenesis and treatment. Hematology Am Soc Hematol Educ Program 605–611. Link: https://bit.ly/3oLs0JN

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
