Archives of Hematology Case Reports and Reviews
Steroid Treatment in Hematologic Patients with COVID-19: Experience of 2 cases
Jlal Bathish, Ayat Zabida, Lihie Eisenberg, Lee Cohen Ben-Meir and Aliza Zeidman*
Cite this as
Bathish J, Zabida A, Eisenberg L, Ben-Meir LC, Zeidman A (2020) Steroid Treatment in Hematologic Patients with COVID-19: Experience of 2 cases. Arch Hematol Case Rep Rev 5(1): 028-033. DOI: 10.17352/ahcrr.000027On March 2020, Hasharon hospital reopened as the first and solely COVID-19 hospital in Israel. For six weeks 52 positive COVID-19 patients were admitted to our ward, Corona B ward. According to previous publications, patients with coexisting hematological condition were more vulnerable to COVID-19 complications. In particular, corticosteroids treatment was reported to reduce the cytokine storm complication of COVID-19. Yet, the literature regarding corticosteroids treatment was controversial at that time. We present two positive COVID-19 hemato-oncologic patients with successful treatment with corticosteroids: a patient with active Chronic Lymphocytic Leukemia (CLL) and a patient with Hodgkin Lymphoma (HL). Epidemiological, clinical, laboratory, imaging and treatment course are discussed along with the relevant literature.
Case number 1
A 69 years old man with a positive COVID-19 PCR test, was admitted to our department on April 4, 2020. Current medical history revealed active Chronic Lymphoid Leukemia (CLL) that was treated with Ibrutinib. The patient also suffered from Obstructive Sleep Apnea (OSA), nephrolithiasis and obesity (BMI 33) (Table 1). He was admitted for persistent fever (38.3ºC) and dry cough, which started 12 days prior to his hospitalization (March 23, 2020). During his pre-hospitalization period he was treated with a 5-days course of oral Levofloxacin, as prescribed by his family physician. Following this treatment the patient had no clinical improvement, and positive COVID-19 PCR test was detected and he was admitted to our ward.
At that time we used 3 categories for illness severity: Mild disease was defined as patient with mild upper respiratory infection or non-severe pneumonia. Moderate disease was defined as patient with pneumonia and one of the following features: Respiratory rate>30 breaths/minute, respiratory distress, or O2 saturation<94% (measured in room air). Severe disease was defined as patient with respiratory failure, Acute Respiratory Distress Syndrome (ARDS), sepsis or shock.
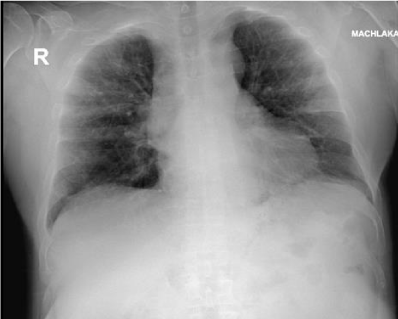
On admission the patient complains of dyspnea and diarrhea. On physical examination he was alert with fever (39.3ºC), increased respiratory rate 30 breaths/minute and decreased O2 saturation- 92% measured in room air). Heart rate was 69 beats/minute and blood pressure was 133/75 mmHg. Laboratory blood tests revealed: White Blood Cell (WBC) count - 19.21 K/micl, thrombocytes-122 K/micl, and increased serum C-Reactive Protein (CRP)-7.3 mg/dL, ferritin-504.8 ng/mL and D-dimer levels -333 ng/mL. PaO2/FiO2 ratio was 118, FiO2 of100. Chest x-ray on admission revealed bilateral patchy consolidation (Figure 1). The patient was categorized as a moderate to severe disease since he had pneumonia, respiratory distress and O2 saturation<94%.
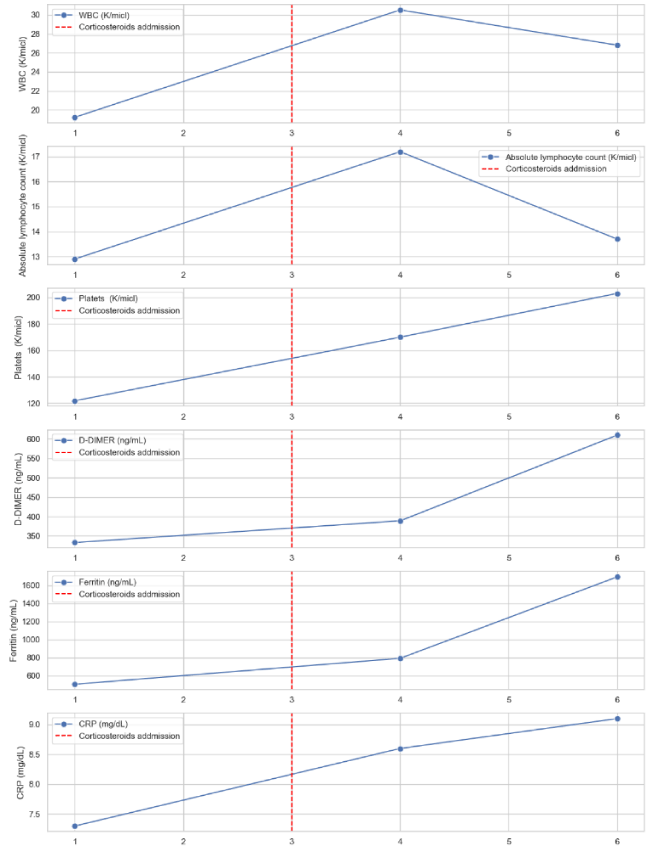
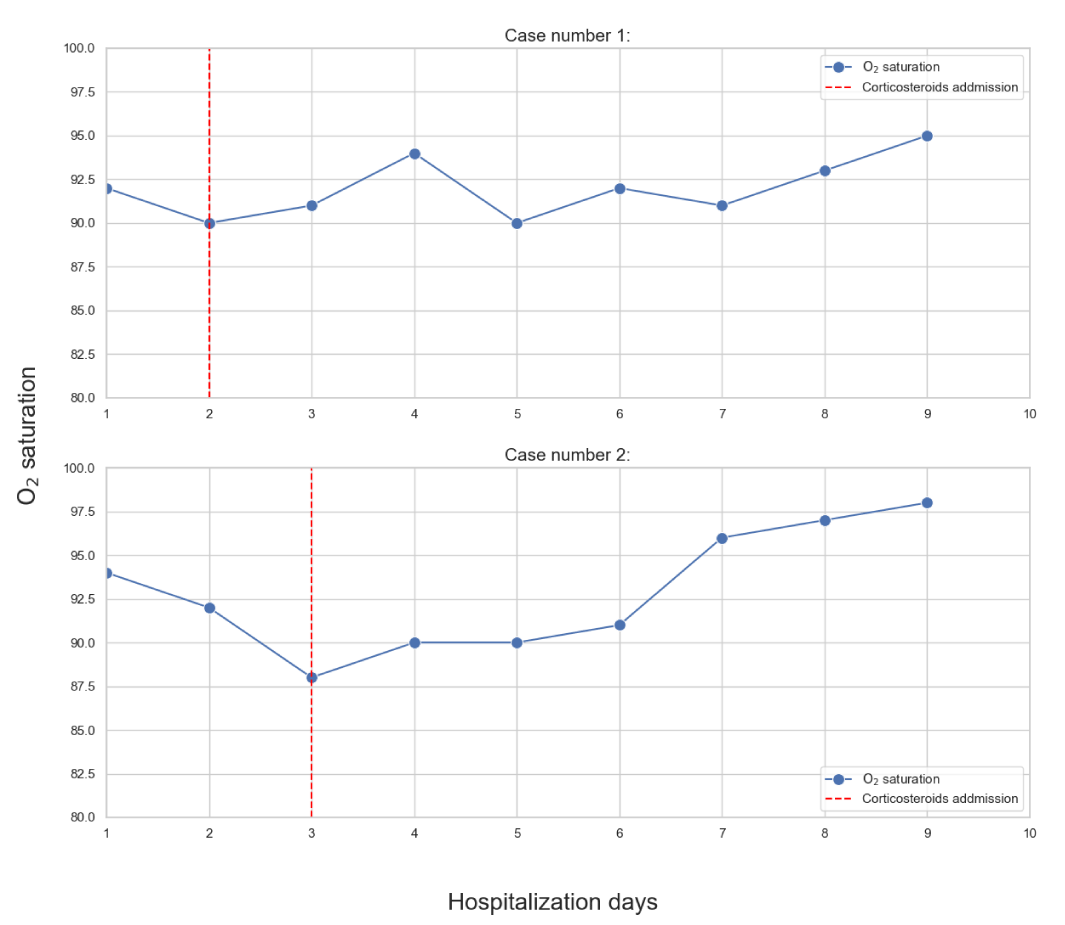
Three days course of Azithromycin 500 mg/day was started followed by five days course of Hydroxychloroquine 200x2 mg/day combined with respiratory physiotherapy but his condition deteriorated with body temperature of 39ºC, O2 saturation decreased to 90% on room air and respiratory rate of 35/min. Laboratory results showed leukocytosis WBC count of 30.53 K/micl with 60% lymphocytes, serum ferritin: 792 ng/mL, serum D-dimer: 389 ng/mL and serum CRP: 8.6 mg/dL. At this stage the patient was in a pre intubation condition and we decided to treat him with high dose parenteral corticosteroids. Solu Medrol (Methylprednisolone sodium succinate) 500 mg/d was given and 24 hours later, the patient’s clinical condition improved remarkably : body temperature decreased to 37.9 ºC His O2 saturation was 93% (in room air) and he did not use the supplemental oxygen anymore. along with laboratory tests improvement: WBC count decreased to 26.82 K/micl) and decrease in lymphocytic count Although all the inflammatory markers were still high for few more days (Figure 2). The treatment was given for 2 more days and on the following days, the patient reported further clinical improvement with milder dry cough and milder dyspnea. On the ninth day of hospitalization the patient was categorized as a mild disease for the first time. Repeated COVID-19 test was still positive, therefore, after ten days of hospitalization, on April 13, 2020 the patient was transferred to a COVID-19 Hotel for further isolation and observation. Follow up clinic 40 days later, the patient is COVID-19 negative, feeling well and continues with hematologic treatment.
Case number 2
A 51 years old man with positive COVID-19 PCR test, was admitted to our department on March 26, 2020. The patient was diagnosed with Hodgkin Lymphoma (HL) in 2017 and was treated with Doxorubicin, Vinblastine and Dacarbazine. He was declared as disease free since 2018. Other comorbidities include hypertension, dyslipidemia, Ischemic Heart Disease (IHD) and ST-elevation myocardial infarction (STEMI). Medical treatment includes Aspirin, Bisoprolol, Ezetimibe, Rosuvastatin and Esomeprazole (Table 1).
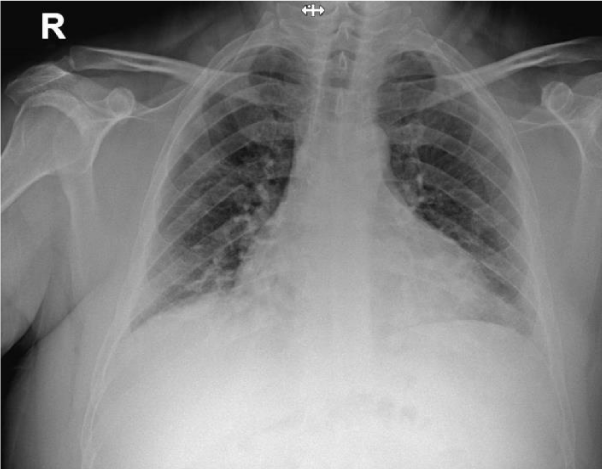
The patient was exposed to a verified positive COVID-19 subject on March 18, 2020 and 4 days later he was tested and found positive for COVID-19 PCR. On admission the patient had fatigue, dry cough and mild dyspnea. He ruled out diarrhea, chills or chest pain. Physical examination revealed normal body temperature of 37.6°C, heart rate 64 beats/minute, respiratory rate 19 breaths/minute and 96% O2 saturation measured in room air (Figure 3). Blood pressure measurement was 121/60 mmHg. Chest x-ray on arrival showed bilateral increased interstitial markings, with no consolidation or pleural effusion (Figure 4). On arrival the patient was categorized as a moderate disease patient.
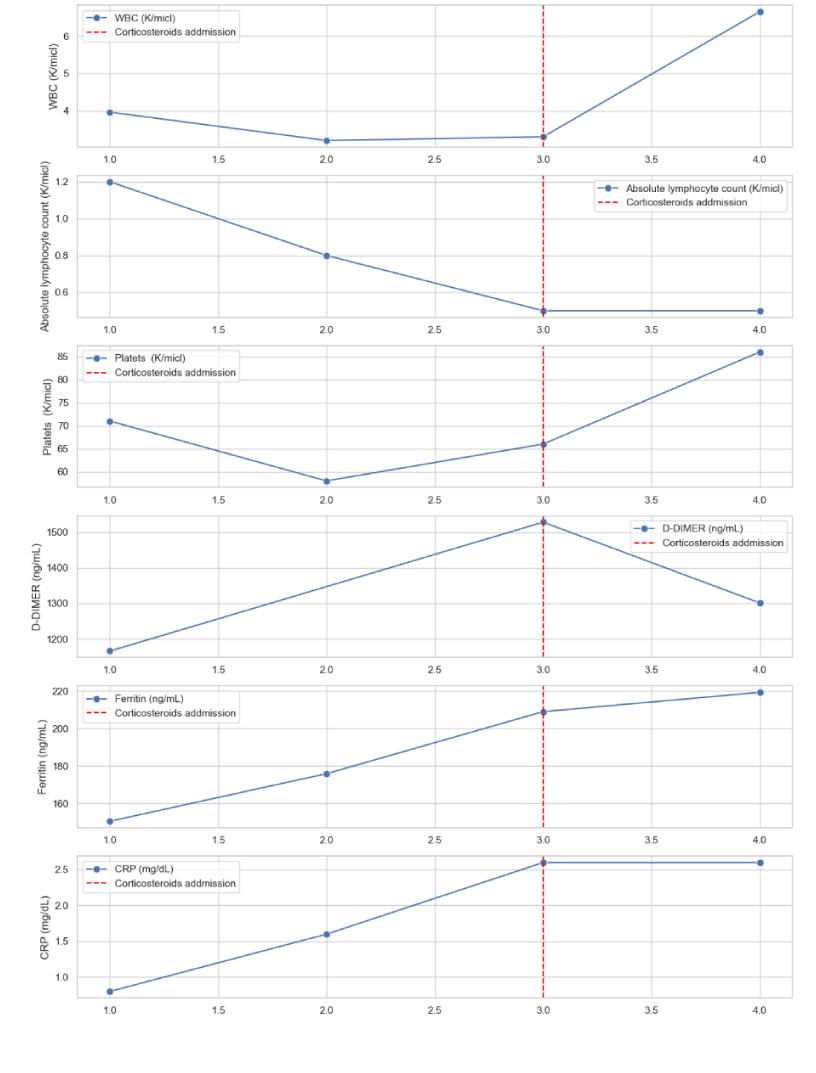
Laboratory tests on admission: leukocytes WBC 3.96 K/micl, thrombocytopenia -71 K/micl, and increased serum CRP 1.8 mg/dL , serum ferritin 150.5 ng/mL and serum D-dimer 1166 ng/mL PaO2/FiO2 ratio was 198, FiO2 of100 (Figure 5).
On the third day of admission the patient’s condition deteriorated: he complains of fatigue, dry cough and dyspnea, respiratory rate was 35 /min and O2 saturation decreased to 90% in air room and no response to supplemental oxygen. Laboratory tests showed severe thrombocytopenia of 66 K/micl and increased inflammatory markers D-dimer 1528 ng/mL and CRP levels 2.6 mg/dL. The patient was categorized as moderate to severe stage and intubation was considered at this stage. As a last conservative treatment at that time, we started parenteral Solu Medrol 500 mg/d and supplemental oxygen, in addition to Levofloxacin 500mg treatment. Following the high dose corticosteroid treatment, there was a remarkable clinical improvement after 48 hours; he reported milder dry cough and no dyspnea, on examination: respiratory rate was 25 and oxygen saturation 94% in room air, laboratory tests improved: decreased serum D-dimer levels (1301 ng/mL) and ferritin levels. He no longer needed supplemental oxygen treatment. Few days later the patient was categorized as mild disease and on April 4, 2020 the patient was discharged home following two consecutive negative COVID-19 PCR tests. 2 weeks later on follow up Corona clinic he reported of weakness but no respiratory consequences.
Discussion
Effective therapies for hospitalized patients with Corona virus are steel in progress and most treatments were based on clinical trials and case reports. Steroid treatment mainly low dose dexamethasone in critical patients is only now established in met analysis that evaluated the role of corticosteroids in the management of COVID-19 patients, has shown that corticosteroids can be used in patients with critical illness with moderate to severe ARDS without the risk of increased mortality [1,2].
On March 2019, most of the publications were based on case reports and there was no consensus regarding corticosteroid treatment. Oncologic patients were reported to be prone to get infected with COVID-19, and present with more severe disease course including hospitalization in the intensive care unit, mechanical ventilation requirement and higher mortality rate [3]. Hemato-oncological patients were reported to have mortality rate up to 40% [4]. Yet, the data on hematologic patients in the aspect of COVID-19 was at that time and still today currently limited. Hasharon hospital reopened as the first and solely COVID-19 hospital in Israel, and 52 patients were admitted to our ward, Corona B ward [5]. Two of these patients had coexisting oncologic hematologic background. Based on A recent study from the hematologic department in Saint-Antoine hospital in Paris, France, followed COVID-19 outcomes in 25 hematologic cancer patients. Among these 25 patients, three patients received corticosteroids treatment during their hospitalization period. All of these three patients had Acute Respiratory Distress Syndrome (ARDS) and bilateral pulmonary opacities imaging. Eventually, two of these three patients deceased and only one of them had survived [6].
Information on corticosteroids treatment in COVID-19 patients is currently limited, and there are controversial findings regarding their use. During the Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS) eruptions, patients were benefited from corticosteroids treatment, which helped in decreasing the inflammatory damage [7]. These immunosuppressant agents were used to overcome the cytokine storm response from an overreaction of the immune system(8). The release of cytokines as TNFα, IL-1β, IL-2, IL-6, IFNα, IFNβ, IFNγ, and MCP-1, provoke free radicals secretion which are the main reason for ARDS and systemic organ failure [9].
Studies reported that high dose steroids were not beneficial for severe lung injury treatment [10]. A retrospective study estimated the outcomes of adjuvant corticosteroid treatment on the prognosis of 244 critically ill COVID-19 patients and found that increased corticosteroid dosage was significantly associated with elevated mortality risk [11]. Nevertheless, using a low-to-moderate dosage of corticosteroids, for a short period has shown to be effective for critically ill COVID-19 patients [12-14].
Conclusion
In this study we described the clinical course of two hematologic patients that admitted to our Corona B ward in Hasharon hospital, the first COVID-19 hospital in Israel. Although data on corticosteroids as a treatment for COVID-19 patients was controversial at that time, we decided to give it as a last resort before intubation, when the available treatments protocols failed to improve their disease course. This decision was based on the known literature regarding the efficacy of corticosteroids treatment in the SARS and MERS eruptions. As the data on corticosteroids treatment for positive COVID-19 hematologic patients is currently limited, future studies that will further investigate these findings are needed.
- The WHO Rapid Evidence Appraisel for COVID 19Therapies (REACT) Working Group, Sterne JAC, Sterne JAC, Murthy S, Diaz JV, Slutsky AS, et al. (2020) Association betweenadministration of systemic corticosteroids and mortality among critically ill patients with COVID 19. A meta- analysis. JAMA 324: 1330-1341. Link: https://bit.ly/2J5iaR8
- Tomazinini BM, MaiaIS, Cavalcanti AB et al. (2020) Effect of Dexamethasone on days alive and ventilator free in patientswith moderate to severeacute respiratory distress syndrome and COVID 19: The CODEX randomized clinical trial. JAMA 324: 1307-1316. Link: https://bit.ly/2IWGs0a
- Liang W, Guan W, Chen R, Wang W, Li J, et al. (2020) Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 21: 335-337. Link: https://bit.ly/3pUvtVB
- Zeidman A, Bathish J, Zabida A, Eisenberg L, Zhalka F, et al. (2020) Clinical experience of COVID-19 patients: disease course and treatment features. G Med Sci 1: 005-015. Link: https://bit.ly/33c8ED2
- Malard F, Genthon A, Brissot E, van de Wyngaert Z, Marjanovic Z, et al. (2020) COVID-19 outcomes in patients with hematologic disease. Bone Marrow Transplant 55: 2180-2184. Link: https://bit.ly/3fqSmLv
- Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, et al. (2003) Clinical Features and Short-term Outcomes of 144 Patients with SARS in the Greater Toronto Area. J Am Med Assoc 289: 2801-2809. Link: https://bit.ly/339KdpC
- Channappanavar R, Perlman S (2017) Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 39: 529-539. Link: https://bit.ly/36ZR2eQ
- Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, et al. (2014) Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124: 188-195. Link: https://bit.ly/3m3to7w
- Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, et al. (2012) Into the Eye of the Cytokine Storm. Microbiol Mol Biol Rev 76: 16-32. Link: https://bit.ly/3l0scjU
- Chang SC (2005) Clinical findings, treatment and prognosis in patients with severe acute respiratory syndrome (SARS). J Chinese Med Assoc 68: 106-107. Link: https://bit.ly/3kUZyjX
- Russell CD, Millar JE, Baillie JK (2020) Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 395: 473-475. Link: https://bit.ly/3fECUeZ
- Lu X, Chen T, Wang Y, Wang J, Zhang B, et al. (2020) Adjuvant corticosteroid therapy for critically ill patients with COVID-19. medRxiv. Link: https://bit.ly/33dpXUi
- Shang L, Zhao J, Hu Y, Du R, Cao B (2020) On the use of corticosteroids for 2019-nCoV pneumonia. Lancet 395: 683-684. Link: https://bit.ly/2UXBYst
- Ye Z, Rochwerg B, Wang Y, Adhikari NK, Murthy S, et al. (2020) Treatment of patients with nonsevere and severe coronavirus disease 2019: an evidence-based guideline. Can Med Assoc J 192: 200648. Link: https://bit.ly/3fpNuX0

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
