Archives of Hematology Case Reports and Reviews
Chronic demyelinating polyneuropathy associated with anti-ganglioside GQ1b antibodies in peripheral T-cell lymphoma
Salem Bahashwan1,2*, Carolyne Croizier1,2, Xavier Moisset1,3, Romain Guièze1,2, Bertrand Evrard1,4, Olivier Tournilhac1,2, Jacques-Olivier Bay1,2 and Richard Lemal1,2
2University hospital of Clermont-Ferrand, Unit of adult cell therapy and clinical hematology, Clermont Ferrand, France
3University Hospital of Clermont-Ferrand, neurology department, Clermont-Ferrand, France
4University hospital of Clermont-Ferrand, department of immunology, Clermont-Ferrand, France
Cite this as
Bahashwan S, Croizier C, Moisset X, Guièze R, Evrard B, et al. (2019) Chronic demyelinating polyneuropathy associated with anti-ganglioside GQ1b antibodies in peripheral T-cell lymphoma. Arch Hematol Case Rep Rev 4(1): 008-010. DOI: 10.17352/ahcrr.000017Malignant lymphoma can cause peripheral neuropathy through various mechanisms. We report the case of 67-year-old man with chronic motor and sensory axonal demyelinating polyneuropathy associated with anti-ganglioside GQ1b antibodies in serum and cerebrospinal fluid (CSF) as an initial presentation of peripheral T-cell lymphoma, not otherwise specified. The patient was treated with chemotherapy for T-cell lymphoma, and achieved complete remission. Motor function recovered completely and sensory function improved. Neurological improvement was concurrent with the disappearance of serum and CSF anti-ganglioside GQ1b antibodies. This is the first report of chronic demyelinating polyneuropathy associated with anti-ganglioside GQ1b antibodies in peripheral T-cell lymphoma.
Introduction
Malignant lymphomas can cause peripheral neuropathy through various mechanisms: directly through infiltration of abnormal lymphocytes into nerve tissue, and indirectly through disease-associated metabolic disorders, infections, immunological responses, complications of treatments (including chemotherapy, radiation and bone marrow transplantation), and paraneoplastic syndromes [1-6].
In non-Hodgkin’s lymphoma (NHL), peripheral neuropathy occurs mainly due to lymphocytic infiltration of nerves adjacent to affected lymph nodes, while in Hodgkin’s lymphoma (HL), direct infiltration is less likely. The cause in HL is more likely to be an autoimmune peripheral neuropathy in the context of the underlying immune disturbance [7-12].
There have been several reports of peripheral neuropathy associated with NHL which were mostly related to B-cell lymphoma. Limited descriptions of neuropathy associated with T-cell lymphoma have been reported, and all were associated with direct infiltration [13-15].
The current report describes the clinical and immunological features of a case of chronic demyelinating autoimmune polyneuropathy with anti-ganglioside GQ1b antibodies associated with peripheral T-cell lymphoma.
Case Report
On admission
A 67-year-old man developed unexplained and progressive bilateral lower limb pain mainly in the ankle joints which was rated 3 out of 10 in severity, “pressing” in nature, exacerbated by movement, relieved by Paracetamol, and associated with mild ankle joint swelling. There was no associated numbness. Two months later, the lower limb pain worsened, the ankle swelling progressed to generalized edema below the knee joints, and new bilateral wrist joint pain similar in character to the lower limb pain appeared. This pain was initially with movement but then became permanent. No fever or weight loss was noted. Upon rheumatologic evaluation, physical examination showed tenderness of ankle and wrist joints, lower limb edema, mild wrist joint swelling, no erythema, no limitation of movement over all joints, no motor weakness or sensory loss, no crepitus or bony knobs, and no muscle wasting or skin rashes. Wrist and ankle X-rays were normal. Initial blood testing showed WBC 9000/mm3, Hb 13 g/dl, platelets 350 000/mm3, neutrophils 6300/mm3, and lymphocytes 530/mm3. CRP, folate, vitamin B12, TSH, T3, and T4 were within normal range. HIV, hepatitis B and C, and syphilis serology tests were negative. An autoimmune test panel was negative (anti-neutrophil cytoplasmic antibodies, antinuclear antibody, extractable nuclear antigen antibodies, anticyclic citrullinated peptide antibody, rheumatoid factor, and Enterobacteriaceae antibody) [1]. 1 mg/kg of prednisone was prescribed to the patient by his family physician, who strongly suspected a rheumatologic problem. 1 mg/kg prednisone was prescribed for 8 weeks, as the patient was already improving, and dose tapering was done over 4 weeks before stopping. Three weeks after starting the prednisone taper, the patient started to have upper and lower limb paresthesia, loss of sensation, and painful cold sensation accompanied by bilateral lower limb weakness and gait disorder. Upon neurological evaluation, clinical examination showed proximal and distal lower limb weakness graded 3/5 (Medical Research Council Manual Muscle Testing scale) and loss of sensation in a stocking-glove pattern. Electromyography (EMG) testing of the radial, ulnar, tibial, and sural nerves was performed, which demonstrated motor and sensory axonal demyelinating polyneuropathy. There was an initial improvement of symptoms after prescribing pregabalin. Three months later, the patient presented to the emergency department complaining of shortness of breath, generalized fatigue, and weight loss of 5 kg in 6 months, without fever or night sweats. Physical examination showed multiple enlarged bilateral cervical, supraclavicular, axillary, and inguinal lymph nodes, which were nontender and rubbery in consistency. The largest node measured was 3×4 cm in the right axillary area. Heart rate was 120 and regular. No murmur or added sounds were noted on cardiac auscultation. Normal vesicular breath sounds were missing bilaterally over the bases and middle lung fields, which were stony dull on percussion. The abdomen was distended with shifting dullness on percussion, and without tenderness, guarding, or rebound tenderness. Splenomegaly was noted, with the spleen edge palpable 5 cm below the costal margin.
Investigations
Serum protein electrophoresis showed a monoclonal IgG level of 0.9 g/l. Protein and glucose levels in the cerebrospinal fluid (CSF) were normal and no abnormal cells were observed on microscopic examination. Onco-neuronal antibodies (Hu, Ma2, amphiphysin, CRMP5, GAD, VGKCs, NMDARs, AMPARs, GABA (B), GlyRs and mGluR5) were negative in the serum and CSF, however anti-ganglioside GQ1b was positive in both (69.84%, 55.1%, respectively).
CT-scanning revealed multiple bulky lymph nodes in the cervical, supraclavicular, axillary, mediastinal, retroperitoneal, and inguinal regions. The bulkiest was in the mediastinum and measured 6.8×4 cm. Splenomegaly, large bilateral pleural effusions, and ascites were also noted. PET imaging showed various levels of hypermetabolic activity in the enlarged lymph nodes and the spleen. The maximum standardized uptake value (SUV) was 22.6, observed in the mediastinum with Deauville score 5.
Cervical excisional lymph node biopsy showed atypical lymphoid cells of small to medium size associated with a significant epithelioid reaction; and pleomorphic cells, small to medium in size with a few large cells, with an increased nucleo-cytoplasmic ratio and frequent mitoses. The cells’ phenotype (CD3+, CD5+, CD2+, CD7+, CD4+, CD8-, CD10-, BCL 6+, PD1+, CXCL13+, Granzyme B-, TIA 1-) was compatible with the diagnosis of peripheral T-cell lymphoma, not otherwise specified (NOS).
Analysis of pleural fluid showed the presence of atypical T-cells which had the same immunological and pathological features of the cells from the cervical lymph node biopsy. Bone marrow biopsy excluded bone marrow involvement by lymphoma.
Treatment and outcome
Prenisone 1 mg/kg was administered immediately after the biopsy with no clinical improvement. After diagnosis, chemotherapy was initiated with prednisone 100 mg per day for 5 days, cyclophosphamide 750 mg/m2 on day 1, doxorubicin 50 mg/m2 on day 1, and etoposide 100 mg/m2 on days 1-3. Eight cycles of treatment were completed and well-tolerated by the patient.
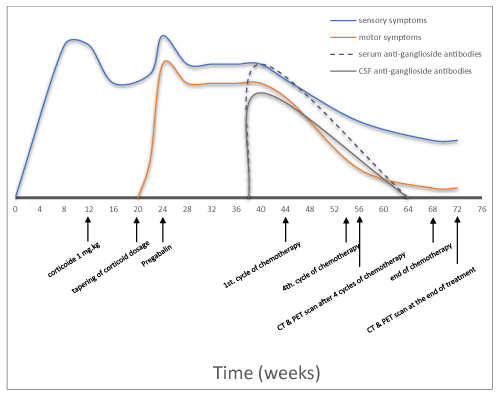
Following four cycles of chemotherapy, CT-scanning showed a partial response of 70%, and PET imaging revealed persistence of hypermetabolic activity in the spleen, with complete metabolic remission in the other sites. Clinical improvement of neuropathy was observed during the course of treatment, with complete motor function recovery and sensory function improvement with persistence of paresthesia. At the end of treatment, complete remission was achieved on CT and PET imaging studies (Deauville score 2). EMG studies showed marked improvement in motor function parameters, but no improvement of sensory parameters. Anti-ganglioside GQ1b antibodies were undetectable in serum and CSF (Figure 1).
Written informed consent was obtained from the patient for publication of this Case Report.
Discussion
Gangliosides are a family of sialylated glycosphingolipids that are abundant in neuronal cell membranes. Anti-ganglioside antibodies bind to presynaptic nerve terminals at the neuromuscular junction, causing synaptic transmission block, which explains the neurological symptoms in this patient with T-cell lymphoma and the presence of anti-ganglioside antibodies in the serum and cerebrospinal fluid (CSF) [16,17].
Peripheral neuropathy can present in patients with NHL, and usually appears during the disease but occasionally represents the initial manifestation of NHL. In that instance, the presentation can be similar to Guillain Barre Syndrome (GBS) or Chronic Inflammatory Demyelinating Polyradiculoneuropathy (CIDP) [7,18-20]. Although there have been several cases of CIDP associated with B-cell lymphoma, very few cases of T-cell lymphoma associated with CIDP have been reported, and all of them were attributed to paraneoplastic syndrome without described antibodies11,18. This is the first report of peripheral neuropathy with anti-ganglioside GD1b antibodies in T-cell lymphoma.
T-cell lymphoma treatment with corticoids and chemotherapy resulted in dramatic improvement of motor neuropathy, which was concomitant with the disappearance of anti-ganglioside GQ1b antibodies in the serum and CSF. However, sensory neuropathy showed little improvement both symptomatically and by EMG testing, raising the possibility that the involved pathologic mechanisms are multifactorial. In addition, sensory improvement could also be delayed, and require active longitudinal monitoring, which is our approach with the patient presented here.
In conclusion, lymphoma with a presentation similar to GBS or CIDP can cause diagnostic confusion, and lymphoma deserves consideration in the presence of any neuropathy, despite its infrequency. Autoimmune neuropathy can be diagnosed in the presence of T cell lymphoma, and chemotherapy can improve the neurological outcome.
- Wada M, Kurita K, Tajima K, Kawanami T, Kato T (2003) A case of inflammatory demyelinating polyradiculoneuropathy associated with T-cell lymphoma. Acta Neurologica Scandinavica 107: 62-66. Link: https://tinyurl.com/yxnolv24
- Kelly JJ, Karcher DS (2005) Lymphoma and peripheral neuropathy: A clinical review. Muscle Nerve 31: 301-313. Link: https://tinyurl.com/yy6kzx9j
- Mori A (2014) Motor-dominant polyneuropathy due to IgM monoclonal antibody against disialosyl gangliosides in a patient with mantle cell lymphoma. Journal of the Neurological Sciences 337: 215-218. Link: https://tinyurl.com/y2pvbwbp
- Viala K, Béhin A, Maisonobe T, Léger J-M, Stojkovic T, et al. (2018) Neuropathy in lymphoma a relationship between the pattern of neuropathy, type of lymphoma and prognosis? Journal of Neurology, Neurosurgery & Psychiatry 79. Link: https://tinyurl.com/y35aj5t2
- Shihashi G, Yagi T, Suzuki S, Seki M, Kohashi S, et al. (2015) Immune-mediated neuropathy with anti-disialosyl IgM antibodies in diffuse large B-cell lymphoma: A case report and literature review. Internal Medicine. 54: 1647-1651. Link: https://tinyurl.com/y2js2589
- Tomita M, Koike H, Kawagashira Y (2013) Clinicopathological features of neuropathy associated with lymphoma. Brain 136: 2563-2578. Link: https://tinyurl.com/yxqzbsf7
- Kelly JJ, Karcher DS (2005) Lymphoma and peripheral neuropathy: A clinical review. Muscle Nerve 31: 301-313. Link: https://tinyurl.com/yy6kzx9j
- Lahrmann H (2001) Acquired neuromyotonia and peripheral neuropathy in a patient with Hodgkin’s disease. Muscle Nerve 24: 834-838. Link: https://tinyurl.com/y5rmdge8
- Hughes RA, Britton T, Richards M (1994) Effects of lymphoma on the peripheral nervous system. J R Soc Med 87: 526-530. Link: https://tinyurl.com/yys8u4u4
- Diaz-Arrastia R (1992) Neurolymphomatosis: a clinicopathologic syndrome re-emerges. Neurology 42: 1136-1141. Link: https://tinyurl.com/y2po7o4j
- Matsubara K, Nigami H, Harigaya H, Osaki M, Baba K (2000) Peroneal mononeuropathy in pediatric Hodgkin’s disease. Journal Leukemia & Lymphoma 40: 205-207. Link: https://tinyurl.com/yyvjxx6g
- O’Neill BP (2003) Neurologic Complications of Hodgkin’s Disease and the Non-Hodgkin’s Lymphomas. Cancer Neurology in Clinical Practice 371-384. Link: https://tinyurl.com/yxb8uyxn
- Wada M, Kurita K, Tajima K, Kawanami T, Kato T (2003) A case of inflammatory demyelinating polyradiculoneuropathy associated with T-cell lymphoma. Acta Neurol Scand 107: 62-66. Link: https://tinyurl.com/y3vmo4sp
- Griggs JJ, Commichau CS, Rapoport AP, Griggs RC (1997) Chronic inflammatory demyelinating polyneuropathy in non-Hodgkin’s lymphoma. Am J Hematol 54: 332-334. Link: https://tinyurl.com/yytud8cc
- Vallat JM (1995) Non-Hodgkin malignant lymphomas and peripheral neuropathies--13 cases. Brain 118: 1233-1245. Link: https://tinyurl.com/y3vtj968
- Garrity PA, Lawrence Zipursky S (1995) Neuronal target recognition. Cell 83: 177-185. Link: https://tinyurl.com/y4fcqeld
- Halstead SK (2008) Eculizumab prevents anti-ganglioside antibody-mediated neuropathy in a murine model. Brain 131: 1197-1208. Link: https://tinyurl.com/y2sjvekn
- Sumi SM, Farrell DF, Knauss TA (1983) Lymphoma and Leukemia Manifested by Steroid-Responsive Polyneuropathy. Arch Neurol 40: 577-582. Link: https://tinyurl.com/y4jjl3at
- Croft PB, Urich H, Wilkinson M (1967) Peripheral neuropathy of sensorimotor type associated with malignant disease. Brain 90: 31-66. Link: https://tinyurl.com/y57vb3fy
- Gherardi R (1986) T-cell lymphoma revealed by a peripheral neuropathy. A report of two cases with an immunohistologic study on lymph node and nerve biopsies. Cancer 58: 2710-2716. Link: https://tinyurl.com/yxkutoek
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
