Archive of Gerontology and Geriatrics Research
Duodenal bleeding in a patient with Covid-19-Related Acute Respiratory Distress Syndrome
Maurizio Gabrielli*, Laura Franza, Maria Chiara Bungaro, Tommaso de Cunzo, Alessandra Esperide, Federico Valletta, Irene Gasparrini, Luca Santarelli, Alessio Migneco, Lorenzo Capaldi and Francesco Franceschi
Cite this as
Gabrielli M, Franza L, Bungaro MC, Cunzo TD, Esperide A, et al. (2020) Duodenal bleeding in a patient with Covid-19-Related Acute Respiratory Distress Syndrome. Arch Gerontol Geriatr Res 5(1): 036-039. DOI: 10.17352/aggr.000024Dear Editor
High incidence of thrombotic complications was observed in patients with Acute Respiratory Distress Syndrome (ARDS) related to Coronavirus Disease 2019 (COVID-19) [1]. The pathophysiology seems related to systemic thrombophilia by hyper-immune reaction, inducing a “cytokine storm” [1].
Yet, gastrointestinal bleeding occurs in 2-3% of ARDS patients. Splanchnic hypo-perfusion is a major cause, due to increased plasma-renin-angiotensin-aldosterone activity, circulating catecholamines levels, and elevated Positive End Expiratory Pression (PEEP) generally used in ARDS [2].
We propose the present case to reflect on the delicate balance between thrombosis and bleeding risk in patients affected by ARDS associated with COVID-19, especially when older, with multiple comorbidities, and undergoing treatments without the support of evidence-based medicine. Indeed, wanting to do “something” for COVID-19 critical patients, many doctors are utilizing treatments with unknown efficacy/safety profile, or never properly studied in critically ill patients: steroids and heparin at dosages higher than prophylactic ones, among others.
A 71-year-old man presented to the emergency department of our hospital on March 27, 2020, for acute respiratory failure. Upon arrival, he was alert, vital signs as follow: blood pressure 104/73 mmHg, heart rate 76 beats/minute, respiratory rate 40 breaths/minute, SpO2 70% in ambient air, body temperature 37.4 °C. Medical history was significant for arterial hypertension, type II diabetes, and previous ischemic stroke. BMI was 31. He was discharged on March 21 from another hospital with diagnosis of COVID-19-related pneumonia without respiratory failure. Home therapy consisted of atenolol, valsartan, hydrochlorothiazide, pravastatin, ticlopidine.
At arrival arterial blood gas analysis at a FIO2 of 0.5 showed: pH 7.51; PaCO2 27.1 mm Hg; PaO2 35.8 mm Hg with a PaO2/FiO2 of 71.6; bicarbonate 27.1 mEq/l; lactate 2.8 mmol/l, arterial O2 saturation 84%. Abnormal laboratory data were: leucocytosis (13.28 k/µl) with lymphopenia (0.860 k/µl); normal haemoglobin (13.3 g/dl); D-dimer (2390 ng/ml); creatinine 1.34 mg/dl; LDH 922 UI/L; C reactive protein 140 mg/l. ECG was substantially normal. Chest Computed Tomography (CT) showed findings of COVID-19-related ARDS. Nasopharyngeal swab confirmed positivity for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2).
The patient was started on Non-Invasive Ventilation (NIV) in Pressure Support Ventilation (PSV) modality (pressure support, PS 12 cmH20; PEEP 8 cmH2O; fO2 50%). After 1 hour, tachypnea ameliorated (28 breaths per minute), pO2/FiO2 increased (128), and blood pressure remained stable. The intensivist did not consider the patient suited for invasive mechanical ventilation suggestingto continue NIV.
In the following days, PS was progressively reduced to 6 cm H20 and PEEP increased to 12 cm H20. The respiratory distress improved (pO2/FiO2 > 150 mmHg at subsequent checks). Pharmacological treatment consisted of fluids, piperacillin/tazobactam, azytromicin, omeprazole, methylprednisolone (1 mg/kg qd) IV; ritonavir, darunavir, hydroxycloroquine, atenolol, valsartan (PO); enoxaparin (1 mg/kg qd SC, sub-therapeutic dosage).
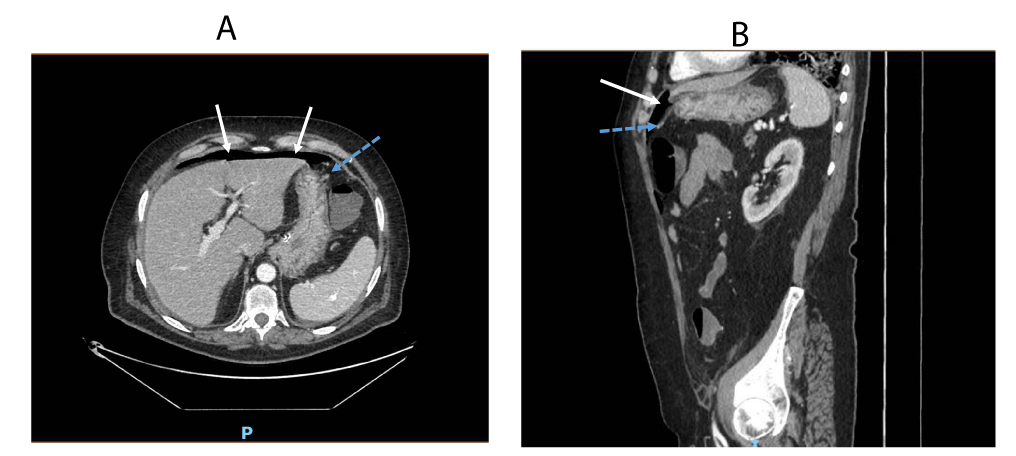
During the night of day 3, the patient reported diffuse abdominal pain, without haemodynamic instability. Urgent blood count showed significant decrease of haemoglobin (9.1 g/dl), low RBC (3.020 mil/µl) and haematocrit (26.7%), normal platelets, persistent mild leucocytosis. Contrast-enhanced abdominal CT revealed duodenal perforation (as demonstrated by a small amount of extra-luminal free air in front of the liver) associated with evident arterial blushing (Figure 1).
Angiography, performed a few hours later, showed stop of the bleeding, therefore excluding the need for artery embolization. After intubation, a small perforation of the anterior surface of the duodenum was identified and sutured during laparotomy surgery. In addition, laparotomy confirmed the absence of active duodenal bleeding, in the presence of abundant amount of corpuscular material in the abdominal cavity, as for recent digestive haemorrage. After surgery, the patient remained intubated, so still on invasive ventilation. Heparin was restarted in 24 hours, but at prophylactic dosage. No re-bleeding was documented, with stable values at serial blood count checks. After initial clinical improvement, he died of suppurative peritonitis few days later.
The haemorrhagic complication of our patient could be due to interaction among three main factors: NIV, steroids, heparin. Guidelines consistently suggest that patients with ARDS and PO2/FiO2 <150 should promptly start on invasive mechanic ventilation, since delays increase mortality. High PEEP and low tidal volumes are suggested [3]. Yet, older patients are at higher risk of complications and of not being extubated following invasive ventilation: CPAP or NIV could, indeed, play a role in this population. However, it is important to remember that administering high pressures during Continuous Positive Airway Pressure (CPAP) or NIV is associated to barotrauma, including gastrointestinal perforation. A “more gentle” ventilation, with low tidal volume, possible only during invasive ventilation, could have prevented duodenal perforation in our patient.
Concerning steroids, there is no clear evidence of their efficacy in SARS-CoV-2-related ARDS [4]. On the other hand, it is well-known that they may give several side-effects, gastrointestinal bleeding or perforation among others. Least but not last the “heparin question”. Many colleagues are using intermediate or therapeutic doses in severe COVID-19 patients, due to reporting of increased risk of venous and arterial thrombosis. Yet, besides case reports and series, the only available (retrospective) study is on 449 Chinese patients with severe COVID-19. No difference was observed in 28-day mortality between patients treated with prophylactic-dose heparin and non-users. The mortality of heparin users was significantly lower only in patients with SIC score ≥ 4, but in our patient SIC score was 2 [5-7]. For this reason, COVID-19-related ARDS should be currently treated with heparin at standard prophylactic dosage.
Finally, the disproportion between the amount of gastrointestinal bleeding and perforation observed in our patient may not have a pure iatrogenic basis. Researches on coagulation pattern of more severe forms of COVID-19 showed that these patients are at higher risk of developing not only thrombotic but also bleeding complications. This is true already within the principal target organ, the lungs: in an autopsy study, pulmonary thrombosis and hemorrhagic lesions were contemporary present in patients with COVID-19. Bleeding could be due to an imbalance in platelets production and disruption, prolonged PT and disseminated intravascular coagulation. Such heterogenous abnormalities of the coagulation system need to be better clarified, in particular to try to understand which patients are more at risk of thrombotic or bleeding complications.
Conclusion
A combination of therapeutic choices, widely used at present in the medical community without solid evidences, possibly both with a peculiar coagulation pattern, may have contributed to death of our patient affected by SARSCoV-2-related ARDS, not due to respiratory failure or thrombotic complications, but to significant gastrointestinal bleeding associated with duodenal perforation.
Below are listed all members of the GEMELLI AGAINST COVID study group:
Abbate Valeria, Acampora Nicola, Addolorato Giovanni, Agostini Fabiana, Ainora Maria Elena, Akacha Karim, Amato Elena, Andreani Francesca, Andriollo Gloria, Annetta Maria Giuseppina, Annicchiarico Brigida Eleonora, Antonelli Mariangela, Antonucci Gabriele, Anzellotti Gian Marco, Armuzzi Alessandro, Baldi Fabiana, Barattucci Ilaria, Barillaro Christian, Barone Fabiana, Bellantone Rocco Domenico Alfonso, Bellieni Andrea, Bello Giuseppe, Benicchi Andrea, Benvenuto Francesca, Berardini Ludovica, Berloco Filippo, Bernabei Roberto, Bianchi Antonio, Biasucci Daniele Guerino, Biasucci Luigi Marzio, Bibbò Stefano, Bini Alessandra, Bisanti Alessandra, Biscetti Federico, Bocci Maria Grazia, Bonadia Nicola, Bongiovanni Filippo, Borghetti Alberto, Bosco Giulia, Bosello Silvia, Bove Vincenzo, Bramato Giulia, Brandi Vincenzo, Bruni Teresa, Bruno Carmine, Bruno Dario, Bungaro Maria Chiara, Buonomo Alessandro, Burzo Livia, Calabrese Angelo, Calvello Maria Rosaria, Cambieri Andrea, Cambise Chiara, Cammà Giulia, Candelli Marcello, Canistro Gennaro, Cantanale Antonello, Capalbo Gennaro, Capaldi Lorenzo, Capone Emanuele, Capristo Esmeralda, Carbone Luigi, Cardone Silvia, Carelli Simone, Carfì Angelo, Carnicelli Annamaria, Caruso Cristiano, Casciaro Francesco Antonio, Catalano Lucio, Cauda Roberto, Cecchini Andrea Leonardo, Cerrito Lucia, Cesarano Melania, Chiarito Annalisa, Cianci Rossella, Cicetti Marta, Cicchinelli Sara, Ciccullo Arturo, Ciciarello Francesca, Cingolani Antonella, Cipriani Maria Camilla, Consalvo Maria Ludovica, Coppola Gaetano, Corbo Giuseppe Maria, Corsello Andrea, Costante Federico, Costanzi Matteo, Covino Marcello, Crupi Davide, Cutuli Salvatore Lucio, D’Addio Stefano, D’Alessandro Alessia, D’alfonso Maria Elena, D’Angelo Emanuela, D’Aversa Francesca, Damiano Fernando, De Berardinis Gian Maria, De Cunzo Tommaso, de Gaetano Donati Katleen, De Luca Giulio, De Matteis Giuseppe, De Pascale Gennaro, De Santis Paolo, De Siena Martina, De Vito Francesco, Del Gatto Valeria, Del Giacomo Paola, Del Zompo Fabio, Dell’Anna Antonio Maria, Della Polla Davide, Di Gialleonardo Luca, Di Giambenedetto Simona, Di Luca Roberta, Di Maurizio Luca, Di Muro Mariangela, Dusina Alex, Eleuteri Davide, Esperide Alessandra, Facheci Daniele, Faliero Domenico, Falsiroli Cinzia, Fantoni Massimo, Fedele Annalaura, Feliciani Daniela, Ferrante Cristina, Ferrone Giuliano, Festa Rossano, Fiore Maria Chiara, Flex Andrea, Forte Evelina, Franceschi Francesco, Francesconi Alessandra, Franza Laura, Funaro Barbara, Fuorlo Mariella, Fusco Domenico, Gabrielli Maurizio, Gaetani Eleonora, Galletta Claudia, Gallo Antonella, Gambassi Giovanni, Garcovich Matteo, Gasbarrini Antonio, Gasparrini Irene, Gelli Silvia, Giampietro Antonella, Gigante Laura, Giuliano Gabriele, Giuliano Giorgia, Giupponi Bianca, Gremese Elisa, Grieco Domenico Luca, Guerrera Manuel, Guglielmi Valeria, Guidone Caterina, Gullì Antonio, Iaconelli Amerigo, Iafrati Aurora, Ianiro Gianluca, Iaquinta Angela, Impagnatiello Michele, Inchingolo Riccardo, Intini Enrica, Iorio Raffaele, Izzi Immacolata Maria, Jovanovic Tamara, Kadhim Cristina, La Macchia Rosa, La Milia Daniele Ignazio, Landi Francesco, Landi Giovanni, Landi Rosario, Landolfi Raffaele, Leo Massimo, Leone Paolo Maria, Levantesi Laura, Liguori Antonio, Liperoti Rosa, Lizzio Marco Maria, Lo Monaco Maria Rita, Locantore Pietro, Lombardi Francesco, Lombardi Gianmarco, Lopetuso Loris, Loria Valentina, Losito Angela Raffaella, Lucia Mothanje Barbara Patricia, Macagno Francesco, Macerola Noemi, Maggi Giampaolo, Maiuro Giuseppe, Mancarella Francesco, Mangiola Francesca, Manno Alberto, Marchesini Debora, Maresca Gian Marco, Marrone Giuseppe, Martis Ilaria, Martone Anna Maria, Marzetti Emanuele, Mattana Chiara, Matteo Maria Valeria, Maviglia Riccardo, Mazzarella Ada, Memoli Carmen, Miele Luca, Migneco Alessio, Mignini Irene, Milani Alessandro, Milardi Domenico, Montalto Massimo, Montemurro Giuliano, Monti Flavia, Montini Luca, Morena Tony Christian, Morra Vincenzina, Moschese Davide, Murace Celeste Ambra, Murdolo Martina, Murri Rita, Napoli Marco, Nardella Elisabetta, Natalello Gerlando, Natalini Daniele, Navarra Simone Maria, Nesci Antonio, Nicoletti Alberto, Nicoletti Rocco, Nicoletti Tommaso Filippo, Nicolò Rebecca, Nicoletti Rocco, Nicolotti Nicola, Nista Enrico Celestino, Nuzzo Eugenia, Oggiano Marco, Ojetti Veronica, Pagano Francesco Cosimo, Paiano Gianfranco, Pais Cristina, Paolillo Federico, Pallavicini Federico, Palombo Andrea, Papa Alfredo, Papanice Domenico, Papparella Luigi Giovanni, Paratore Mattia, Parrinello Giuseppe, Pasciuto Giuliana, Pasculli Pierpaolo, Pecorini Giovanni, Perniola Simone, Pero Erika, Petricca Luca, Petrucci Martina, Picarelli Chiara, Piccioni Andrea, Piccolo Annalisa, Piervincenzi Edoardo, Pignataro Giulia, Pignataro Raffaele, Pintaudi Gabriele, Pisapia Luca, Pizzoferrato Marco, Pizzolante Fabrizio, Pola Roberto, Policola Caterina, Pompili Maurizio, Pontecorvi Flavia, Pontecorvi Valerio, Ponziani Francesca, Popolla Valentina, Porceddu Enrica, Porfidia Angelo, Porro Lucia Maria, Potenza Annalisa, Pozzana Francesca, Privitera Giuseppe, Pugliese Daniela, Pulcini Gabriele, Racco Simona, Raffaelli Francesca, Ramunno Vittoria, Rapaccini Gian Ludovico, Richeldi Luca, Rinninella Emanuele, Rocchi Sara, Romanò Bruno, Romano Stefano, Rosa Federico, Rossi Laura, Rossi Raimondo, Rossini Enrica, Rota Elisabetta, Rovedi Fabiana, Rubino Carlotta, Rumi Gabriele, Russo Andrea, Russo Andrea, Sabia Luca, Salerno Andrea, Salini Sara, Salvatore Lucia, Samori Dehara, Sandroni Claudio, Sanguinetti Maurizio, Santarelli Luca, Santini Paolo, Santolamazza Danilo, Santoliquido Angelo, Santopaolo Francesco, Santoro Michele Cosimo, Sardeo Francesco, Sarnari Caterina, Saviano Angela, Saviano Luisa, Scaldaferri Franco, Scarascia Roberta, Schepis Tommaso, Schiavello Francesca, Scoppettuolo Giancarlo, Sedda Davide, Sessa Flaminio, Sestito Luisa, Settanni Carlo, Siciliano Matteo, Siciliano Valentina, Sicuranza Rossella, Simeoni Benedetta, Simonetti Jacopo, Smargiassi Andrea, Soave Paolo Maurizio, Sonnino Chiara, Staiti Domenico, Stella Claudia, Stella Leonardo, Stival Eleonora, Taddei Eleonora, Talerico Rossella, Tamburello Elio, Tamburrini Enrica, Tanzarella Eloisa Sofia, Tarascio Elena, Tarli Claudia, Tersali Alessandra, Tilli Pietro, Timpano Jacopo, Torelli Enrico, Torrini Flavia, Tosato Matteo, Tosoni Alberto, Tricoli Luca, Tritto Marcello, Tumbarello Mario, Tummolo Anita Maria, Vallecoccia Maria Sole, Valletta Federico, Varone Francesco, Vassalli Francesco, Ventura Giulio, Verardi Lucrezia, Vetrone Lorenzo, Vetrugno Giuseppe, Visconti Elena, Visconti Felicia, Viviani Andrea, Zaccaria Raffaella, Zaccone Carmelina, Zelano Lorenzo, Zileri Dal Verme Lorenzo, Zuccalà Giuseppe.
- Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, et al. (2020) High Risk of Thrombosis in Patients with Severe SARS-CoV-2 Infection: A Multicenter Prospective Cohort Study. Intensive Care Med 46: 1089-1098. Link: https://bit.ly/33snx5a
- Siddiqui F, Ahmed M, Abbasi S, Avula A, Siddiqui AH, et al. (2019) Gastrointestinal Bleeding in Patients with Acute Respiratory Distress Syndrome: A National Database Analysis. J Clin Med Res 11: 42-48. Link: https://bit.ly/33qFnVY
- Poston JT, Patel BK, Davis AM (2020) Management of Critically Ill Adults With COVID-19. JAMA 323: 1839-1841. Link: https://bit.ly/3fw1NHK
- Li X, Xu S, Yu M, Wang K, Tao Y, et al. (2020) Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol 146: 110-118. Link: https://bit.ly/3gubuIj
- Tang N, Bai H, Chen X, Gong J, Li D, et al. (2020) Anticoagulant Treatment Is Associated with Decreased Mortality in Severe Coronavirus Disease 2019 Patients with Coagulopathy. J Thromb Haemost 18: 1094-1099. Link: https://bit.ly/3a0H3qK
- Jiang H, Liu L, Guo T, Wu Y, Ai L, et al. (2019) Improving the safety of CAR-T cell therapy by controlling CRS-related coagulopathy. Ann Hematol 98: 1721-1732. Link: https://bit.ly/2Xw44fU
- Giannis D, Ziogas IA, Gianni P (2020) Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol 127: 104362. Link: https://bit.ly/3i8ghPP
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
