Annals of Environmental Science and Toxicology
Cytotoxicity, genotoxicity, and mutagenicity of the active pharmaceutical ingredient nevirapine and a nevirapine-based drug on the plant species Allium cepa
Juliana Souki Diniz1*, Gabriel de Souza-Silva1, Clessius Ribeiro de Souza1, Leonardo Alvarenga de Paula Freitas2, Ana Luísa Souki Parreira3, Brennda Rocha Pena1, Marcos Paulo Gomes Mol2 and Micheline Rosa Silveira1
2Research and Development Department, Ezequiel Dias Foundation, Brazil
3Federal University of Sao Joao del Rei, Brazil
Cite this as
Diniz JS, Souza-Silva GD, De Souza CR, De Paula Freitas LA, Souki Parreira AL, et al. (2023) Cytotoxicity, genotoxicity, and mutagenicity of the active pharmaceutical ingredient nevirapine and a nevirapine-based drug on the plant species Allium cepa. Ann Environ Sci Toxicol 7(1): 025-033. DOI: 10.17352/aest.000067Copyright
© 2023 Diniz JS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The toxicity of the active pharmaceutical ingredient and nevirapine-based drug at analytical concentrations was evaluated under laboratory conditions, using Allium cepa seeds as a model. The germination index of the negative control was 86.8 ± 2.1. The concentrations of 6.42 and 9.54 mg/L of the active pharmaceutical ingredient and 11.20 mg/L of the nevirapine-based drug showed a statistically higher germination index than the negative control. We found that the root growth of the negative control was 1.7 ± 0.6 mm and that the root growth was statistically lower than the negative control at concentrations of 9.54 and 17.73 mg/L of active pharmaceutical ingredient and 5.48, 11.20, and 17.68 mg/L of the drug. The mitotic index of negative control and methyl methanesulfonate were 7.4 ± 2.7 and 12.8 ± 4.5, respectively. At a concentration of 17.68 mg/L of the nevirapine-based drug, the mitotic index of 12.7 ± 2.7 was statistically higher than the negative control and like the methyl methanesulfonate, which indicated that nevirapine was cytotoxic at this concentration. At all concentrations tested the chromosome abnormality indices were statistically higher than negative control, suggesting that nevirapine was genotoxic. The mutagenicity index of the negative control was 0.2 ± 0.3. At concentrations of 6.42, 9.54, and 17.73 mg/L of the active pharmaceutical ingredient and 17.68 mg/L of the nevirapine-based drug, the mutagenicity index was statistically higher than the negative control, indicating that nevirapine was mutagenic for A. cespa at these concentrations. The chromosomal adhesion was the most frequent chromosomal aberration in the groups exposed to nevirapine, suggesting that it has an aneugenic effect on the A. cepa species.
Introduction
Nevirapine is an antiretroviral from the non-nucleoside reverse transcriptase inhibitor class used in combination therapy for the treatment of Human Immunodeficiency Virus (HIV) infection. It is an active pharmaceutical ingredient used in the manufacture of medicines used by about 35% of the world’s population, especially in low-income countries, as one of the therapeutic options available at low cost [1]. The nevirapine acts on the metabolic pathway responsible for DNA transcription, inhibiting reverse transcriptase and preventing the virus from multiplying.
Nevirapine is a dipyridodiazepine with a dipyridyl chemical structure, also found in some herbicides such as Diquat® (9,10-dihydro-8a,10a-diazoniaphenanthrene) [2]. This structure is a precursor of radical species that operate as electron acceptors in oxidative metabolism [3], inducing oxidative metabolism [4]. Radical species can generally induce chromosomal alterations, break the Deoxyribonucleic Acid (DNA) double-strand, and oxidize sulfhydryl groups without catalysts [5]. The cytotoxic, genotoxic, and mutagenic potential of nevirapine deserves to be evaluated through ecotoxicological tests [6], which can trigger the production of reactive oxygen species when observing the chemical structure in the nevirapine molecule [7].
Although nevirapine is a poorly water-soluble (90 mg/L), its high stability against light and heat [2], and low environmental biodegradability [8], contribute to its persistence in water, soil, and sediments [9,10]. In countries with a high prevalence of people living with HIV (PLHIV), environmental studies have identified the presence of nevirapine residue in surface water, at concentrations of 0.5 to 1.5 µg/L [9,11] and 1480 ng/L and in sediments, with concentrations of 9.5 µg/L in the dissolved fraction and 3214 µg/Kg in the undissolved fraction [12].
Contamination with micropollutants such as active pharmaceutical ingredients is a concern of the World Health Organization (WHO) regarding the human use of medicinal plants worldwide. Based on this information, Akenga, et al. [13] studied the plant absorption of molecules, including nevirapine, using hydroponic lettuce seeds (Lactuca sativa). Given the lack of knowledge of the effects on future generations and based on the numerous adverse effects described in the literature in children exposed to antiretrovirals in the perinatal period [14]. Therefore, they chose the plant species Allium cepa [15,16] as a study model, an alternative to the methodological difficulties and bioethical conflicts for conducting cytogenotoxicity assays in animal models.
Indeed, the assay with A. cepa to evaluate the genotoxicity of chemical compounds is consolidated in the [17,18], medicinal plants [19-25] and pharmaceuticals [26,27], showing sensitivity and good correlation compared to other systems, such as mammals [28]. This model has been widely used for monitoring basins [29], environmental pollutants [30-33], and industrial [34-36].
The presence and impacts of nevirapine residues in the environment justify carrying out studies like this one, since they imbalance numerous species and harm human health. In this sense, the present study aimed to investigate the potential cytotoxic, genotoxic, and mutagenic effect of the active pharmaceutical ingredient nevirapine and a nevirapine-based drug on the experimental model A. cepa to contribute with different responses to the impact of this residue in the environment.
Materials and methods
We adopted the methodology of the study by Caritá, et al. [34] adapted for laboratory conditions to conduct this study, standardizing the experimental variables such as the (i) use of seeds, (ii) preparation of solutions, (iii) exposure method, (iv) assay time, (v) radicle fixation, (vi) preparation and staining of slides with histological sections, and (vii) preparation of permanent slides.
Chemical substances
Anhydrous form active pharmaceutical ingredient nevirapine (batch C5028-12009M), raw material with a purity content of 100.49%, manufactured by Zhejiang Huahai Pharmaceutical Co. Ltd (Linhai, China) was employed to prepare an active pharmaceutical ingredient nevirapine stock solution. Tablets of the product Nevirax® 200 mg (batch 14030011) with a purity content of 100.20%, manufactured by Fundação Ezequiel Dias (FUNED) (Belo Horizonte, Brazil), were pulverized to prepare a stock suspension of the drug nevirapine. The three concentrations of the active pharmaceutical ingredient working solutions and the three-drug working suspensions were analyzed by a validated analytical method using the United States Pharmacopeia (USP) anhydrous chemical reference substance (CRS) NVP (batch G0M270). Methyl methanesulfonate reagents (Sigma-Aldrich, 66-27-3), trifluralin (Sigma-Aldrich, 1582-09-8), Carnoy reagent solutions, 1% acetic carmine and Schiff reagent were provided by the Public Health/Water Laboratory of the Faculty of Pharmacy of the Federal University of Minas Gerais. The reagents and solvents used in this study were analytical grades and concentrations approved by FUNED’s quality control for all preparation steps.
Solutions, suspensions, and reagents
A standard stock solution was prepared with 15.82 mg of anhydrous chemical reference substance nevirapine, solubilized in a 25 mL volumetric flask containing 10 mL of acetonitrile and 15 mL of purified water (Millipak® 20 millipore Direct – Q® 3UV). From this solution, three other working standard nevirapine solutions were prepared at concentrations of 12.66, 31.64, and 63.28 mg/L for the calibration curve.
For the tests, a solution and a stock suspension, respectively, of the active pharmaceutical ingredient and the drug were prepared to obtain, after dilution, working solutions and suspensions at concentrations of 5, 10, and 20 mg/L of nevirapine in purified water, having its pH adjusted to 6.9 ± 0.1. Such concentrations are close to 10 mg/L, similar to the plasma concentration of nevirapine found in humans [37]. An aqueous suspension of trifluralin (0.019 mg/L) [38,39], an aqueous solution of methyl methanesulfonate (4 x 10-4 mol/L) [40] were prepared for the positive controls, and purified water with adjusted pH of 6.9 ± 0.1 was used for the negative control.
Stains for preparing permanent slides
Following the protocol by Caritá, et al. [34] with adaptations, Carnoy’s fixative was prepared with ethanol and glacial acetic acid at a ratio of 3:1 (v/v) for immediate use for the fixation and conservation of radicles. Acetic carmine 1% was prepared by solubilizing 1 g of carmine in 100 mL of 45% acetic acid, followed by a boiling process for two to three hours and filtration.
For the Schiff reagent, 1.5 g of basic fuchsin and 4.5 g of sodium metabisulfite were solubilized under stirring in 300 mL of heated water (50 °C) and 45 mL of 1 mol/L hydrochloric acid. Then, the solution was heated and stirred for fifteen minutes and stored in the dark for 24 hours. After standing, 1 g of activated carbon was added to the solution and filtered, separating 10 mL aliquots in a dropper flask. Finally, the aliquots were stored in the refrigerator in a dark bottle, wrapped in aluminum foil, and kept from light. A new aliquot was used for each working day.
Analytical determination of nevirapine
To determine the analytical concentration of nevirapine, at the initial time of the test, an aliquot of three mL of each working solution/suspension sample (active pharmaceutical ingredient and nevirapine-based drug) was filtered through a 0.45 μm filter and transferred to Falcon tubes for further quantification by ultra-performance liquid chromatography (UHPLC) (Shimadzu Nexera-Prominence®) coupled to a photodiode detector (model SPD-M20A), both Shimadzu (Columbia, USA), using the analytical method under the chromatographic conditions described by Diniz, et al. [41] [Separation was done using a 2.0 × 100 mm, 2.2 µm particle diameter C18 column (ShimPack XR® UHPLC). The nevirapine peak was monitored at 214 nm and occurred 11 minutes after the start of the run.
Test organism
Allium cepa (Baia Periforme variety) seeds, batch 42011-52, from ISLA PAK, germination index of 89% and purity of 100% acquired in the retail trade of Belo Horizonte, Southeast of Brazil.
Seed germination test
The adapted test by Christofoletti, et al. [42] was used, using 25 seeds of A. cepa continuously exposed to four mL of the working solution/suspension of the active pharmaceutical ingredient or nevirapine-based drug in each Petri dish autoclaved with filter paper. The positive (trifluralin and methyl methanesulfonate) seeds and negative controls (purified water) received four mL of the respective component. Then, the plates were closed, coated with polyvinyl chloride (PVC) film, and incubated in an oven (Fanem - Biochemical Oxygen Demand) for 120 hours in the absence of light at a temperature of 22 ± 2 °C. Four plates were prepared, totaling at least 100 seeds for each concentration in each group. After incubation, the germination index was calculated for each concentration of each group per the percentage of germinated seeds against the total number of exposed seeds.
Root growth
The Onwuamah, et al. [14] root growth assessment method was adapted and used in this study. After 120 hours, the radicles emerging from the seeds were evaluated for phenotypic aspects, such as (i) color, (ii) texture, and (iii) growth alterations (folds, swollen mass, and bifid radicles). Then, with a caliper (with a scale of 0.05 mm – 1/128”) (Disma), the radicle length in millimeters (mm) was measured for each seed that developed in each group, except methyl methanesulfonate (for biological safety reasons, the radicles were not manipulated for measurement).
Slide preparation: staining and fixation
The methodology described by Caritá, et al. [34] was adopted. After incubation for 120 hours, ten rootlets from each plate were collected, sectioned, and fixed with Carnoy (3:1) one hour before the start of the slide preparation. Before the preparation, the radicles were washed with purified water and dried. The Feulgen reaction [43] was performed in a beaker containing 5 mL of 1 mol/L Hydrochloric Acid (HCl) and heated in a water bath at 60 °C for 11 minutes. Then, the radicles were quickly washed to stop hydrolysis, dried, and placed inside an amber glass bottle containing the Schiff reagent. After two hours of incubation in the dark, the excess reagent was removed with a paper towel.
The meristematic region (region of cell division) of the rootlets and the F1 region (region of root elongation or growth, located immediately after the meristematic region and preceding the region of cell maturation or differentiation) of each group were separated and cut with a scalpel. Then each region was covered with a coverslip, and 1% acetic carmine was dripped onto the sample. The excess solution was removed, and the slide was exposed to the flame rapidly for two seconds. In the end, the prepared slides were immersed in liquid nitrogen for fixation and conservation of the samples for later evaluation in an optical microscope (Eclipse E200) in the 40 times objective.
Assessment of the mitotic index
At least 500 cells from the meristematic region of each slide were analyzed, making a total of 5,000 cells analyzed from each group. Following the criteria of Caritá and Marin-Morales [40], the stage of cell division was identified by the phase of the nucleus of each cell (such as interphase or phases of mitosis - prophase, metaphase, anaphase, and telophase). The mitotic index was calculated through the percentage of dividing cells (nucleus in mitotic phases) against the total number of analyzed cells (nucleus in mitotic and interphase phases).
Evaluation of chromosome aberrations and nuclear alterations in the meristematic region
The test was adapted from the Grant [44,45] and Caritá, et al. [34] protocol. At least 5,000 cells from each group were analyzed in the meristematic region for the presence of binuclei, polyploid cells, C-metaphase, micronuclei, microcells, cell sprouts, breakage, loss, chromosomal adhesion, multipolar anaphases, bridges, and anaphase or telophase delays. Following the criteria of Caritá and Marin-Morales [40], the stage of cell division was identified by the phase of the nucleus of each cell (such as interphase or phases of mitosis - prophase, metaphase, anaphase, and telophase). The mitotic index was calculated through the percentage of dividing cells (nucleus in mitotic phases) against the total number of analyzed cells (nucleus in mitotic and interphase phases).
The chromosome aberration index was obtained by the percentage of cells with chromosomal alterations against the total number of cells analyzed for each group. The nuclear alteration index was obtained by the percentage of cells with alterations in the nucleus (minicells, micronuclei, and sprouts) against the total number of cells analyzed for each group.
Assessment of the mutagenicity index
At least 3,000 cells were analyzed for each group in the F1 region for micronuclei and sprouts. Briefly, the mutagenicity index was obtained by the percentage of cells with micronuclei and sprouts against the total number of analyzed cells of each concentration in each group [34,46].
Statistical analysis
Statistical analysis was performed using the free software R, version 4.0.3. Initially, a Shapiro-Wilk test was conducted to verify data normality. Then, from the results obtained in each group, a two-by-two Mann-Whitney comparison was implemented for non-parametric data, as it was more sensitive. Values of p < 0.05 (i.e., 95% confidence) were considered a statistically significant difference.
Results and discussion
In this study, the active pharmaceutical ingredient and the nevirapine-based drug showed similar analytical concentrations (Table 1), which facilitated the comparison between them regarding the possible interference of the excipients in the observed results. The germination index of the seed lot used in the tests met expectations based on the negative control germination index (86.8 ± 2.1). The germination index of the seeds evaluated the nevirapine cytotoxicity on A. cepa
The germination of A. cepa seeds exposed to a trifluralin of 48.2 ± 6.2 was statistically lower than the negative control (p = 0.0294). Usually, herbicides inhibit the protein activity of cells, inhibiting development and causing injury or death of the organism [47]. Trifluralin belongs to the dinitroaniline group and acts selectively as a mitosis disruptor, inhibiting cell division in meristematic tissues, with aneugenic effects [38,39].
As for the seeds exposed to methyl methanesulfonate, a germination index of 91.8 ± 1.9 was observed, therefore statistically higher than the negative control (p = 0.0396). methyl methanesulfonate acts on the guanine and adenine nitrogenous bases of DNA by adding or replacing alkyl groups, which causes incorrect base pairing, blocking cell replication, and permanent changes in the genetic material [48]. This macroscopic result agrees with the expected for methyl methanesulfonate as a positive control because, at the concentrations used, it did not prevent seed germination and allowed the elongation of the radicles to observe genetic alterations in A. cepa and its use in the comparison of genotoxicity and mutagenicity.
At concentrations of 6.42 ± 0.58 and 9.54 ± 0.87 mg/L of active pharmaceutical ingredient and 11.20 ± 1.13 mg/L of the nevirapine-based drug, germination index was statistically higher than the negative control (germination index 95.2 ± 2.5; p = 0.0284; 95.2 ± 2.5; p = 0.0294 and 96.8 ± 2.6; p = 0.0284, respectively) and similar to methyl methanesulfonate (p > 0.05). We observed that about 10% inhibition of germination (germination index 79.8 ± 2.1; p = 0.0284) at a concentration of 5.48 ± 0.44 mg/L of the nevirapine-based drug was evidenced when compared to the negative control. The concentrations of 17.73 ± 1.31 mg/L of the active pharmaceutical ingredient and 17.68 ± 1.2 mg/L of the drug showed a germination index similar to the negative control (germination index 84.2 ± 2.5 and 86.8 ± 4.5; p > 0.05), which allows us to state that nevirapine did not inhibit germination at these concentrations, but we cannot say about the feasibility of the organism and its generations.
Concerning growth, the negative control radicles showed homogeneous mass and color and without morphological abnormalities, with a mean growth of 1.7 ± 0.6 mm in 120 test hours, while bifid, twisted, brittle roots and presence of tumors (bulging roots) when exposed to concentrations of active pharmaceutical ingredient and nevirapine-based drug.
Table 2 shows the root growth of the groups. At concentrations 9.54 ± 0.87 and 17.73 ± 1.31 mg/L of active pharmaceutical ingredient and 5.48 ± 0.44; 11.20 ± 1.13 and 17.68 ± 1.29 mg/L of the drug, the root growth was statistically lower than the negative control, reaching a 25% reduction in the concentration 5.48 ± 0.44 mg/L of the drug (root growth 1.5 ± 0.6 mm; p = 0.0108; 1.4 ± 0.6 mm; p = 0.0180; 1.3 ± 0.6 mm; p = 0.0002; 1.4 ± 0.5 mm; p = 0.0017 and 1.4 ± 0.6 mm; p = 0.0000, respectively). The root growth of 1.7 ± 0.5 was similar to the negative control (p > 0.05) only at the concentration of 6.42 ± 0.58 mg/L of API.
These results are corroborated by Onwuamah, et al. [14]. These authors used the syrup pharmaceutical form, but active pharmaceutical ingredient results that would allow comparing the possible interferences of syrup and excipients in the determination of the effective inhibition concentration of 50% (EC50%) of A. cepa root growth were not presented. It is noteworthy that the concentrations of the isolated active pharmaceutical ingredient and the drug in the present study were within the range tested by Onwuamah, et al. [14]. However, they were lower than the EC50% of 24.63 mg/L of nevirapine determined by them.
However, these macroscopic results did not allow a complete inference about the cytotoxic potential of the substances tested because damage to the genetic material may have occurred even without a significant change in germination and root growth, making it necessary to assess the integrity of the proliferative process through histological sections in the meristematic cells [49]. Thus, the mitotic index was used to confirm evidence of cytotoxicity [50].
The mitotic index (MI) of negative control and methyl methanesulfonate were 7.4 ± 2.7 and 12.8 ± 4.5, respectively. At the concentration of 17.68 ± 1.29 mg/L of the nevirapine-based drug, the mitotic index of 12.7 ± 2.7 was statistically higher than the negative control (p = 0.0029) and similar to the methyl methanesulfonate (p > 0.05), which indicated that nevirapine was cytotoxic at this concentration. The mitotic index of 8.5 ± 4.0 observed at the concentration of 6.42 ± 0.58 mg/L of the active pharmaceutical ingredient was the only one similar to the negative control (p > 0.05) and that differed from the methyl methanesulfonate (p = 0.0185), which suggested that nevirapine did not cause disturbances in the proliferative process of A. cepa at this concentration. On the other hand, at the concentration of 17.68 ± 1.29 mg/L of the drug, the mitotic index of 12.7 ± 2.7 was statistically higher than the negative control (p = 0.0029) and similar to the methyl methanesulfonate (p > 0.05), indicating that nevirapine can be considered cytotoxic at this concentration. This result was similar to that of Onwuamah, et al. (2014), who observed a significant reduction in the mitotic index after exposure of onion bulb roots to a nevirapine syrup at a concentration of 12.32 mg/L of nevirapine; however, without the comparison with the alkylating agent.
Fiskejo [16] and Krüger [51] consider that root growth and mitotic index can be endpoints to be observed for the evaluation of cytotoxicity. We observed that, in this study, the macroscopic findings of root growth corresponded to the mitotic index at concentrations of 6.42 ± 0.58 mg/L of active pharmaceutical ingredient (non-cytotoxic) and 17.68 ± 1.29 mg/L of the drug (cytotoxic).
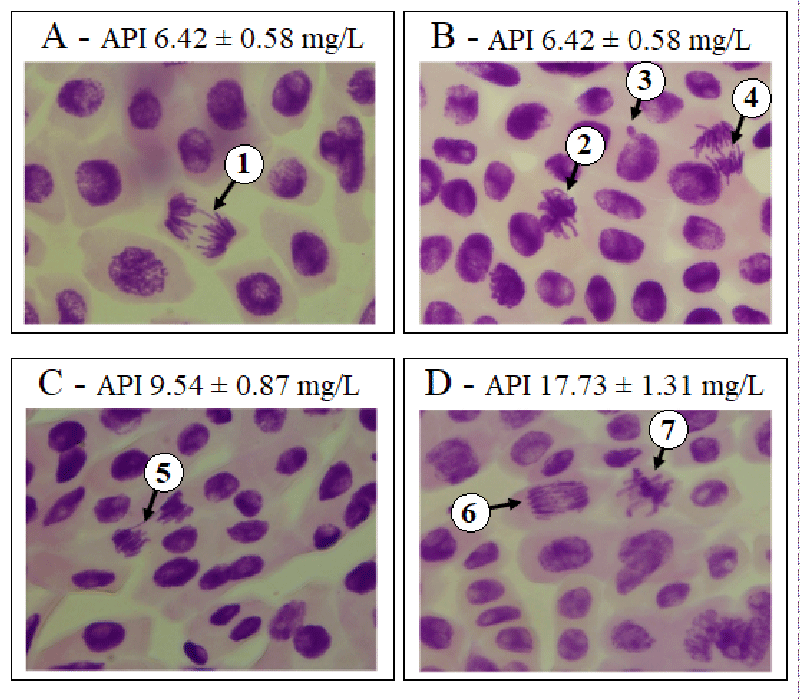
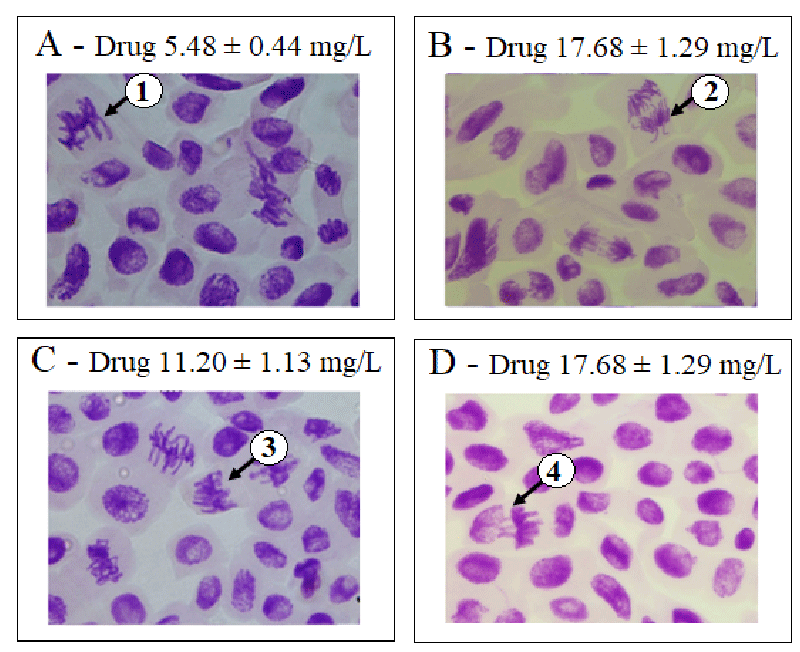
Genotoxicity was assessed using the chromosome aberration index. We found that the chromosome abnormality indices of the negative control, methyl methanesulfonate, and trifluralin were 0.2 ± 0.4; 0.9 ± 2.1, and 1.0 ± 3.3, respectively. At the concentrations of 6.42 ± 0.58; 9.54 ± 0.87; 17.73 ± 1.31 mg/L of active pharmaceutical ingredients and 5.48 ± 0.44; 11.20 ± 1.13 and 17.68 ± 1.29 mg/L of the nevirapine-based drug, chromosome aberration index were statistically higher than the negative control and similar to methyl methanesulfonate and trifluralin (p > 0.05), suggesting that nevirapine showed genotoxic potential at all concentrations tested chromosome aberration index 1.4 ± 0.6; p = 0.0003; 1.7 ± 1.3; p = 0.0016; 1.1 ± 1.3; p = 0.0086; 0.8 ± 1.5; p = 0.0202; 0.6 ± 0.4; p = 0.0419; 2.0 ± 1.3; p = 0.0011, respectively). It is important to note that the highest chromosome aberration index was observed at the concentration of 17.68 ± 1.29 mg/L of the nevirapine-based drug, besides the most significant inhibition of root growth and the highest mitotic index. Abnormalities such as loss, breakage, and adhesion of chromosomes were observed at this concentration. Figures 1 and 2 show examples of chromosome aberrations identified in the groups exposed to nevirapine.
A percentage of 1.2 ± 1.7 of chromosome adhesions was verified in the negative control. At concentrations of 6.42 ± 0.58; 9.54 ± 0.87 and 17.73 ± 1.31 mg/L of active pharmaceutical ingredient and 5.48 ± 0.44; 11.20 ± 1.13 and 17.68 ± 1.29 mg/L of the nevirapine-based drug, the percentages of chromosome adhesions were higher than the negative control (chromosome adhesions 7.9 ± 3.3; p = 0.0003; 10.3 ± 5.5; p = 0.0013; 6.6 ± 5.3; p = 0.0072; 6.6 ± 6.0); p = 0.0112; 3.7 ± 2.6; p = 0.0319; 9.3 ± 5.2; p = 0.0008, respectively). This abnormality was the most frequently observed in this study. This result is corroborated by Onwuamah, et al. [14], who also observed the predominance of chromosome adhesions against other types of abnormalities when A. cepa was exposed to nevirapine.
According to Kurás [52], adhesions result from an alteration in the proportion between histones and other proteins responsible for the organization of nuclear chromatin that increase the adhesion, inducing atypical metaphases and anaphases, chromosomal bridges and inhibiting cytokinesis, forming binucleated cells, making them indications of aneugenic effects of the test substance [35,28].
Indeed, the percentages of adhesions in the groups exposed to nevirapine at the concentrations tested were similar (p > 0.05) to trifluralin (chromosome adhesions 10.5 ± 13.0). This substance acts in cell division phases, selectively inhibiting tubulin (the enzyme responsible for the formation of microtubules), inhibiting seed germination, and the formation of new cells in the rootlet and stem [46]. Thus, the predominance of chromosome adhesions observed in the groups exposed to nevirapine suggests that it has an aneugenic effect and trifluralin [53]. Based on its mechanism of action, nevirapine, like trifluralin, may act as an enzyme inhibitor in metabolic pathways responsible for synthesizing proteins in charge of mitotic spindle activities.
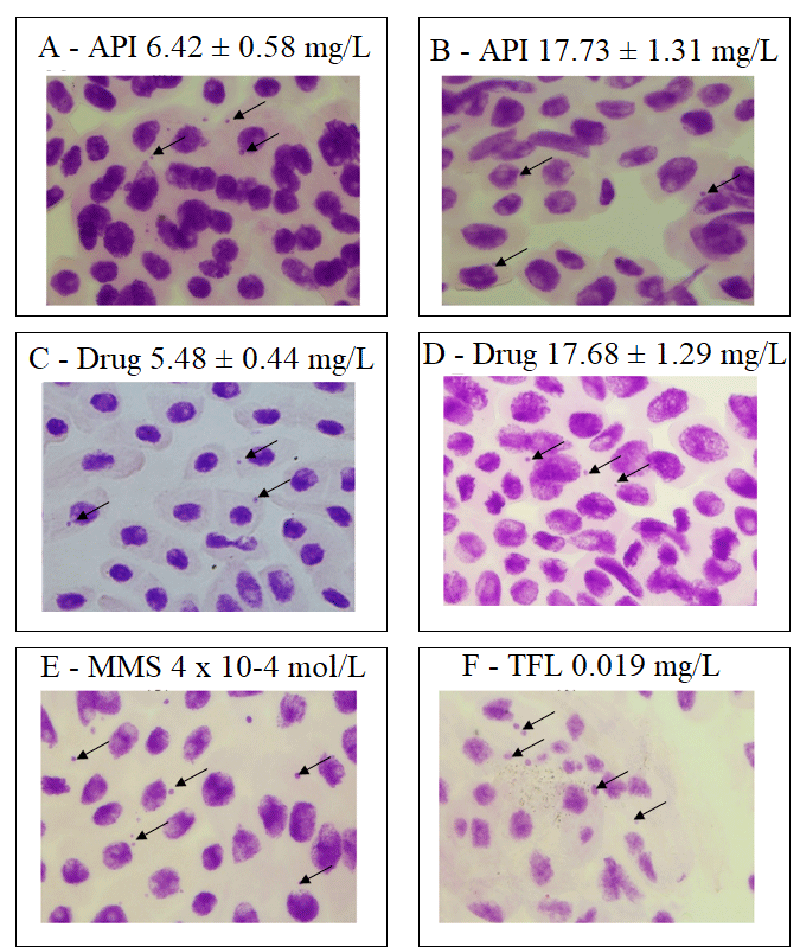
The histological analysis illustrates the presence of micronuclei in the meristematic region (Figure 3) as a possible aneugenic effect related to the induction of radicular species related to the dipyridyl structure of nevirapine, which may have contributed to damage to the nuclear genetic material that tends to be expelled from inside the cell in as sprouts or micronuclei. An essential contribution of this study was the nevirapine mutagenicity index (Table 3), which was investigated in the meristematic and the F1 regions.
The negative control mutagenicity index was 0.2 ± 0.3. At concentrations of 6.42 ± 0.58; 9.54 ± 0.87 and 17.73 ± 1.31 mg/L of the active pharmaceutical ingredient and 17.68 ± 1.29 mg/L of the nevirapine-based drug, the mutagenicity index were statistically higher than the negative control (mutagenicity index 1.8 ± 1.4; p = 0.0013; 1.3 ± 0.9; p = 0.0017; 1.0 ± 0.8; p = 0.0003; 0.5 ± 0.3; p = 0.0237, respectively), indicating that nevirapine was mutagenic for A. cepa at these concentrations. At concentrations of 5.48 ± 0.44 and 11.20 ± 1.13 mg/L of the drug, there was no difference regarding the negative control mutagenicity index. Thus, nevirapine was not mutagenic in these cases. Sprouts and micronuclei were observed in the F1 region. However, the most frequently observed abnormality was the presence of micronuclei at all nevirapine concentrations. We could not identify any minicell in any of the slides.
Micronuclei are generally located peripherally in cells and originate from chromosomal breaks and losses and polyploidy not repaired by the cell [54]. Sprouts and micronuclei are formed by expelling excess genetic material, which may originate in the meristematic region. When not repaired by the cell, the genotoxic effects spread and can be observed in the first generation of daughter cells in the F1 region of the radicles, modifying the rate of nuclear alterations [38,39,54].
Indeed, in Table 3, we observed that the nuclear abnormality index (sprouts, micronuclei) in the meristematic region of the negative control was 0.4 ± 0.3 and that, at concentrations of 6.42 ± 0.58; 9.54 ± 0.87, and 17.73 ± 1.31 mg/L of active pharmaceutical ingredient and 5.48 ± 0.44; 11.20 ± 1.13, and 17.68 ± 1.29 mg/L of the nevirapine-based drug, the nuclear abnormality index were statistically higher than the negative control (nuclear abnormality index of 1.4 ± 0.9; p = 0.0003; 1.0 ± 0.4; p = 0.0015; 1.3 ± 0.9; p = 0.0094; 1.0 ± 0.7; p = 0.0199; 0.8 ± 1.0; p = 0.0417, and 1.3 ± 0.7; p = 0.0011, respectively), replicating for all mutagenicity indices calculated for the F1 region, except for concentrations 5.48 ± 0.44 and 11.20 ± 1.13 mg/L of the nevirapine-based drug.
Although in the Ames test with Salmonella sp. and Escherichia coli, in the mammalian cell mutation test (Chinese hamster ovary - CHO / HGPRT cells), and in the mouse bone marrow micronucleus assay, nevirapine was not considered mutagenic or clastogenic by the International Agency of Research on Cancer (IRTC) [55], the present study showed that the plant species A. cepa was sensitive to exposure to this drug.
The results showed cytotoxic, genotoxic, and mutagenic effects that can be reproduced in other species, especially when considering the nevirapine bioconcentration shown for the lettuce (Lactuca sativa) plant species [13]. In this sense, one should be concerned about the possible environmental residues of nevirapine contaminating medicinal plants and other vegetables, which deserve attention when controlling their release and mitigation in water and effluent treatment plants, which may affect directly and indirectly human health.
Nevirapine solubility was one of the principal challenges for the study. However, the validity of the results obtained is in the design of the study certifying the actual concentration of nevirapine to which the seeds were exposed for germination and root growth, besides the application of protocols internationally accepted that allowed confirming the results obtained. Although only one nevirapine-based drug was evaluated, this study showed that the concentration of 9.54 ± 0.87 mg/L of the active pharmaceutical ingredient promoted a similar response to the concentration of 11.20 ± 1.13 mg/L of the nevirapine-based drug, indicating that the excipients did not interfere with the toxicity observed for nevirapine only at this concentration.
The concentration of 6.42 ± 0.58 mg/L of active pharmaceutical ingredient was not considered cytotoxic based on germination index, root growth, and mitotic index but was genotoxic and mutagenic based on chromosomal abnormality and mutagenicity indices. Genotoxicity was observed at all concentrations tested, although only at concentrations of 6.42 ± 0.58; 9.54 ± 0.87, and 17.73 ± 1.31 mg/L of the active pharmaceutical ingredient and the concentration of 17.68 ± 1.29 mg/L of the nevirapine-based drug, the mutagenic potential was observed. nevirapine at a concentration of 17.68 ± 1.29 mg/L of the drug was considered cytotoxic, genotoxic, and mutagenic.
Conclusion
The drugs have therapeutic effects, but they can also cause dose-dependent or not toxic effects, such as cytotoxicity, genotoxicity, and mutagenicity demonstrated for A. cepa and that can threaten other species. The continuous exposure of the environment to micropollutants such as nevirapine can be mitigated with investments in sanitary sewage, effluent treatment plants, awareness, and health education for the proper disposal of drug residues. Besides nevirapine’s genotoxic and mutagenic effects shown in this work, several other micropollutants can contribute to measuring the environmental risk resulting from industrial production and human environmental consumption of medicines.
- TenoRes Study Group. Global epidemiology of drug resistance after failure of WHO recommended first-line regimens for adult HIV-1 infection: a multicentre retrospective cohort study. Lancet Infect Dis. 2016 May;16(5):565-575. doi: 10.1016/S1473-3099(15)00536-8. Epub 2016 Jan 29. Erratum in: Lancet Infect Dis. 2016 Jun;16(6):636. Erratum in: Lancet Infect Dis. 2018 Jan;18(1):21. PMID: 26831472; PMCID: PMC4835583.
- Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, Li Q, Shoemaker BA, Thiessen PA, Yu B, Zaslavsky L, Zhang J, Bolton EE. PubChem 2019 update: improved access to chemical data. Nucleic Acids Res. 2019 Jan 8;47(D1):D1102-D1109. doi: 10.1093/nar/gky1033. PMID: 30371825; PMCID: PMC6324075.
- Roede JR, Miller GW. Diquat. Encyclopedia of Toxicology. 2014; 2: 202-204.
- Guo X, Liu F, Deng J, Dai P, Qin Y, Li Z, Wang B, Fan A, Wang Z, Zhao Y. Electron-Accepting Micelles Deplete Reduced Nicotinamide Adenine Dinucleotide Phosphate and Impair Two Antioxidant Cascades for Ferroptosis-Induced Tumor Eradication. ACS Nano. 2020 Nov 24;14(11):14715-14730. doi: 10.1021/acsnano.0c00764. Epub 2020 Nov 6. PMID: 33156626.
- Alkadi H. A Review on Free Radicals and Antioxidants. Infect Disord Drug Targets. 2020;20(1):16-26. doi: 10.2174/1871526518666180628124323. PMID: 29952268.
- Del Valle LG, Hernández RG, Ávila JP. Oxidative stress associated to disease progression and toxicity during antiretroviral therapy in human immunodeficiency virus infection. Journal of Virology & Microbiology. 2013: 1- 15.
- Liang Y, Liang N, Yin L, Xiao F. Cellular and molecular mechanisms of xenobiotics-induced premature senescence. Toxicol Res (Camb). 2020 Oct 1;9(5):669-675. doi: 10.1093/toxres/tfaa073. PMID: 33178427; PMCID: PMC7640926.
- Nyirenda J, Mwanza A, Lengwe C. Assessing the biodegradability of common pharmaceutical products (PPs) on the Zambian market. Heliyon. 2020 Oct 21;6(10):e05286. doi: 10.1016/j.heliyon.2020.e05286. PMID: 33117900; PMCID: PMC7581924.
- Adeola AO, Forbes PBC. Antiretroviral Drugs in African Surface Waters: Prevalence, Analysis, and Potential Remediation. Environ Toxicol Chem. 2022 Feb;41(2):247-262. doi: 10.1002/etc.5127. Epub 2021 Jul 16. PMID: 34033688.
- Walenga NJ, Nomngongo PN. Occurrence of pharmaceuticals in the environmental waters: African and Asian perspectives. Environmental Chemistry and Ecotoxicology. 2022; 4: 50-66. doi: 10.1016/j.enceco.2021.11.002.
- K'oreje KO, Demeestere K, De Wispelaere P, Vergeynst L, Dewulf J, Van Langenhove H. From multi-residue screening to target analysis of pharmaceuticals in water: development of a new approach based on magnetic sector mass spectrometry and application in the Nairobi River basin, Kenya. Sci Total Environ. 2012 Oct 15;437:153-64. doi: 10.1016/j.scitotenv.2012.07.052. Epub 2012 Aug 28. PMID: 22935682.
- Kairigo P, Ngumba E, Sundberg LR, Gachanja A, Tuhkanen T. Contamination of Surface Water and River Sediments by Antibiotic and Antiretroviral Drug Cocktails in Low and Middle-Income Countries: Occurrence, Risk and Mitigation Strategies. Water. 2020; 12(5): 1376.
- Akenga P, Gachanja A, Fitzsimons MF, Tappin A, Comber S. Uptake, accumulation and impact of antiretroviral and antiviral pharmaceutical compounds in lettuce. Sci Total Environ. 2021 Apr 20;766:144499. doi: 10.1016/j.scitotenv.2020.144499. Epub 2020 Dec 24. PMID: 33418261.
- Onwuamah CK, Ekama SO, Audu RA, Ezechi OC, Poirier MC, Odeigah PG. Exposure of Allium cepa root cells to zidovudine or nevirapine induces cytogenotoxic changes. PLoS One. 2014 Mar 5;9(3):e90296. doi: 10.1371/journal.pone.0090296. PMID: 24599327; PMCID: PMC3943917.
- Fiskesjö G. The allium test in wastewater monitoring. Environmental Toxicology and Water Quality. 1993; 8(3): 291-298.
- Fiskesjö G. Allium test II: Assessment of a chemical's genotoxic potential by recording aberrations in chromosomes and cell divisions in root tips of Allium cepa L. Environmental Toxicology and Water Quality. 1994; 9(3): 235-241.
- Bonciu E, Firbas P, Fontanetti CS, Wusheng J, Karaismailoğlu MC, Liu D. An evaluation for the standardization of the Allium cepa test as cytotoxicity and genotoxicity assay. Caryologia. 2018; 71(3): 191-209.
- Parvan LG, Leite TG, Freitas TB, Pedrosa PAA, Calixto JS, Agostinho LA. Bioassay with Allium cepa reveals genotoxicity of herbicide with flumioxazin. Revista Pan-Amazônica de Saúde. 2020; 11.
- Bagatini MD, Silva ACFD, Tedesco SB. Use of the Allium cepa test system as a bioindicator of genotoxicity of medicinal plant infusions. Rev. bras. pharmacogn. 2007; 17(3): 444-447.
- Camparoto ML, Teixeira RO, Mantovani MS, Vicentini VEP. Effects of Maytenus ilicifolia Mart. and Bauhinia candicans Benth infusions on onion root-tip and rat bone-marrow cells. Genetics and Molecular Biology. 2002; 25(1): 85- 89.
- Carmo LR, Leal LS, Ribeiro LR. Allium cepa e teste do Micronúcleo como bioindicadores de citogenotoxicidade em extratos aquosos de plantas medicinais. Brazilian Journal of Development. 2020; 6(10): 82419- 82430.
- Knoll MF, Silva ACF, Canto-Dorow TS, Tedesco SB. Effects of Pterocaulon polystachyum DC. (Asteraceae) on onion (Allium cepa) root-tip cells. Genetics and Molecular Biology. 2006; 29(3): 539-542.
- Neves KADS, Oliveira JVA, Teixeira AZA. use of bioassay with Allium cepa L. for the cytotoxicity assessment of Croton urucurana Baill. Ciências e Educação. 2020; 6(12): 07.
- Dos Reis HS. Absence of cytotoxic and genotoxic effects of the aqueous extract of Stryphnodendron adstringens (Barbatimão) bark using Allium cepa test. Biota Amazônia. 2020; 10(1): 20 - 23.
- Guedes CM, Lima MVS, Barbosa MDR, Luz JMM, Rocha AJ, Silva TS, Silva APS, Peron AP. Toxicidade de Ginkgo biloba L., sem aditivação e associado a excipientes artificiais, em tecido meristemático. Stellata Editora. Itajubá, Minas Gerais. 2020; 1-29.
- Grisolia CK, Takahashi CS. Evaluation of mutagenic effect of the antihypertensive drug methyldopa (Aldomet) on mammalian systems in vivo and in vitro and on Allium cepa. Mutat Res. 1991 Feb;259(2):127-32. doi: 10.1016/0165-1218(91)90046-o. PMID: 1994243.
- Raji JI, Onwuamah CK, Odeigah PGC. Artemisinin-Based Combination Therapy Depressed Mitosis and Induced Chromosome Aberration in Onion Root Cells. J Toxicol. 2018 Aug 23;2018:4671326. doi: 10.1155/2018/4671326. PMID: 30210539; PMCID: PMC6126092.
- Leme DM, Marin-Morales MA. Allium cepa test in environmental monitoring: a review on its application. Mutat Res. 2009 Jul-Aug;682(1):71-81. doi: 10.1016/j.mrrev.2009.06.002. Epub 2009 Jul 2. PMID: 19577002.
- Rodrigues GZP, Dalzochio T, Gehlen G. Use of bioassay with Allium cepa L. and physicochemical and microbiological analyzes to assess the quality of Rio da Ilha, RS, Brazil. Acta Toxicológica Argentina. 2016; 24(2): 97-104.
- Cabrera GL, Rodriguez DM. Genotoxicity of soil from farmland irrigated with wastewater using three plant bioassays. Mutat Res. 1999 May 19;426(2):211-4. doi: 10.1016/s0027-5107(99)00070-6. PMID: 10350600.
- Hoshina MM, Marin-Morales MA. Micronucleus and chromosome aberrations induced in onion (Allium cepa) by a petroleum refinery effluent and by river water that receives this effluent. Ecotoxicol Environ Saf. 2009 Nov;72(8):2090-5. doi: 10.1016/j.ecoenv.2009.07.002. Epub 2009 Jul 31. PMID: 19647317.
- Leme DM, de Angelis Dde F, Marin-Morales MA. Action mechanisms of petroleum hydrocarbons present in waters impacted by an oil spill on the genetic material of Allium cepa root cells. Aquat Toxicol. 2008 Jul 30;88(4):214-9. doi: 10.1016/j.aquatox.2008.04.012. Epub 2008 May 4. PMID: 18556073.
- Soodan R, Katnoria J, Nagpal A. Allium cepa Root Chromosomal Aberration Assay: An Efficient Test System for Evaluating Genotoxicity of Agricultural Soil. International Journal of Science and Research. 2014; 3(8): 245-250.
- Caritá R, Mazzeo DEC, Marin-Morales MA. Comparison of the toxicogenetic potential of sewage sludges from different treatment processes focusing agricultural use. Environ Sci Pollut Res Int. 2019 Jul;26(21):21475-21483. doi: 10.1007/s11356-019-05453-y. Epub 2019 May 24. PMID: 31127510.
- Leme DM, Marin-Morales MA. Chromosome aberration and micronucleus frequencies in Allium cepa cells exposed to petroleum polluted water--a case study. Mutat Res. 2008 Jan 31;650(1):80-6. doi: 10.1016/j.mrgentox.2007.10.006. Epub 2007 Oct 24. PMID: 18068420.
- Matsumoto ST, Mantovani MS, Malaguttii MIA, Dias AL, Fonseca IC, Marin-Morales MA. Genotoxicity and mutagenicity of water contaminated with tannery effluents, as evaluated by the micronucleus test and comet assay using the fish Oreochromis niloticus and chromosome aberrations in onion root-tips. Genetics and Molecular Biology. 2006; 29(1): 148-158.
- Viramune. 2019. Tablets/(nevirapine) oral suspension, U.S. prescribing information. Boehringer Ingelheim Pharmaceuticals, Inc. Ridgefield, CT 06877 USA. 2019. 108 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5ec05500-6333-4bd0-ac83-464fad0d5162
- Fernandes TCC, Mazzeo DEC, Marin-Morales MA. Mechanism of micronuclei formation in polyploidizated cells of Allium cepa exposed to trifluralin herbicide. Pesticide Biochemistry and Physiology. 2007; 88(3): 252-259.
- Fernandes TC, Mazzeo DE, Marin-Morales MA. Origin of nuclear and chromosomal alterations derived from the action of an aneugenic agent--Trifluralin herbicide. Ecotoxicol Environ Saf. 2009 Sep;72(6):1680-6. doi: 10.1016/j.ecoenv.2009.03.014. Epub 2009 May 5. PMID: 19419762.
- Caritá R, Marin-Morales MA. Induction of chromosome aberrations in the Allium cepa test system caused by the exposure of seeds to industrial effluents contaminated with azo dyes. Chemosphere. 2008 Jun;72(5):722-5. doi: 10.1016/j.chemosphere.2008.03.056. Epub 2008 May 20. PMID: 18495201.
- Diniz JS, Freitas LAP, Vaz ICD, Barbosa FAR, Mol MPG, Magalhães SME, Silveira MR. The toxic effects of the antiretroviral nevirapine and a nevirapine-based drug for aquatic organisms. Research, Society and Development. 2022; 11(2): e19211225014.
- Christofoletti CA, Francisco A, Fontanetti CS. Biosolid soil application: Toxicity tests under laboratory conditions. Applied and Environmental Soil Science. 2012: 1-9.
- Mello MLS, Vidal BC. The Feulgen reaction: A brief review and new perspectives. Acta Histochem. 2017 Jul;119(6):603-609. doi: 10.1016/j.acthis.2017.07.002. Epub 2017 Jul 21. PMID: 28739089.
- Grant WF. Chromosome aberration assays in Allium. A report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutat Res. 1982 Nov;99(3):273-91. doi: 10.1016/0165-1110(82)90046-x. PMID: 7177154.
- Grant WF. The present status of higher plant bioassays for the detection of environmental mutagens. Mutat Res. 1994 Oct 16;310(2):175-85. doi: 10.1016/0027-5107(94)90112-0. PMID: 7523890.
- Ma TH, Xu Z, Xu C, McConnell H, Rabago EV, Arreola GA, Zhang H. The improved Allium/Vicia root tip micronucleus assay for clastogenicity of environmental pollutants. Mutat Res. 1995 Apr;334(2):185-95. doi: 10.1016/0165-1161(95)90010-1. PMID: 7885371.
- Lancaster S, Jugulam M, Jones JF. Herbicide Mode of Action. C715, Kansas State University, K-STATE Research and Extension, USA. 2021.
- Vonarx EK, Mitchell HL, Karthikeyan R, Chatterjee I, Kunz BA. DNA repair in higher plants. Mutation Research. 1998; 400(1-2): 187-200.
- Cuchiara CC, Borges CS, Bobrowski VL. Allium cepa test system as a bio indicator of cytogenotoxicity of watercourses. Revista Ciencia y Tecnología Agropecuaria. 2012; 6(1): 33-38.
- Mercado SAS, Caleño JDQ. Cytotoxic evaluation of glyphosate, using Allium cepa L. as bioindicator. Sci Total Environ. 2020 Jan 15;700:134452. doi: 10.1016/j.scitotenv.2019.134452. Epub 2019 Oct 4. PMID: 31629268.
- Krüger RA. Toxicity and genotoxicity analysis of pesticides used in agriculture using bioassays with Allium cepa. 58 f. Dissertation (Master in Environmental Quality). Feevale University Center. New Hamburg - RS.
- Kurás L. Characterization of protein–DNA association in vivo by chromatin immunoprecipitation. In: Dickson RC, Mendenhall MD, editors. Signal transduction protocols, methods in molecular biology. Totowa: Humana Press Inc. 2004; 284:147–162.
- Fernandes TCC, Pizano MA, Marin-Morales MA. Characterization, Modes of Action and Effects of Trifluralin: A Review. Herbicides - Current Research and Case Studies in Use. London. 2013; 489-515.
- Grover IS, Kaur S. Genotoxicity of wastewater samples from sewage and industrial effluent detected by the Allium root anaphase aberration and micronucleus assays. Mutat Res. 1999 May 19;426(2):183-8. doi: 10.1016/s0027-5107(99)00065-2. PMID: 10350595.
- Vetmedica, B.I. 2015. Safety data sheet United States nevirapine. Version 1. https://www.bivetmedica.com/sites/default/files/MSDS/nevirapine-sds-us.pdf
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
