Annals of Environmental Science and Toxicology
Effects of Fluoride on Respiration and Photosynthesis in Plants: An Overview
Krishna Kumar*, Arup Giri*, Prince Vivek, Kalaiyarasan T and Bhuvnesh Kumar
Arup Giri, Defence Institute of High Altitude Research (DIHAR), DRDO Leh-Ladakh, Jammu & Kashmir, India; Email: arupsatadal@gmail.com
Cite this as
Kumar K, Giri A, Vivek P, Kalaiyarasan T, Kumar B (2017) Effects of Fluoride on Respiration and Photosynthesis in Plants: An Overview. Ann Environ Sci Toxicol 2(1): 043-047. DOI: 10.17352/aest.000011Among all the halides, Fluoride (F) caused most severe adverse effects on plants through air, soil, and water, exposure. Besides the nutritional importance of this element, after its desirable limits in the plants, it caused adverse effects on plants through interfering with various physic-biochemical parameters; with or without any visible symptoms of injury. Due to higher level of fluorides in plants, it also causes less development of plants through affecting several pathways associated with photosynthesis, respiration, protein synthesis, carbohydrate metabolism, and nucleotide synthesis. Photosynthesis and respiration are the most important process in the plants to acquire the basic energy and their utilization. Fluoride has great effective importance on these to pathways. On this view, the present review critically discusses the toxic effects on respiration and photosynthesis of agricultural crops and trees.
Introduction
Fluoride is the most important member of halides group. In halide group effect of Chloride and Iodide on organism have been well studied [1-3], but less is understood about the biological importance of fluoride. The fluoride is smallest and most electronegative anion in the halide series. Fluoride has its unique chemical and biochemical properties for the size and reactivity, but the mechanisms of cell signaling for the fluoride are incompletely characterized. Fluoride is ubiquitously present in the environment that means it is found in soil, water and air [4]. It is a highly abundant element in the earth crust approx 0.32 g/kg [5, 6]. The distribution of fluoride in soil and water is variable depending on location. In soil, range of fluoride concentrations are from ten to thousands of parts per million (ppm) [6]. In natural water sources, the concentrations range from l25 μM to 100 mM (<0.5 to >2,000 ppm; 1 ppm > 55 μM) depending if the water is in contact with high levels of fluoride-containing minerals [7,8]. In groundwater, fluoride concentrations is highest among the any anion [4], fluoride is one of the most important phytotoxic air pollutants. Fluoride (F) toxicity on terrestrial plants has been studied and has been clearly demonstrated in the entire scientific manner like laboratory, greenhouse and controlled field plot experiments [9]. Injury to vegetation due to high concentration fluoride, commonly gradual accumulation of in the plant tissue over a period of time [10]. It has the capability to induce the abnormal morphological symptoms like chlorosis, tip and marginal necrosis etc [11]. Sensitivity will be different in various species and varieties to fluoride are reported. Fluoride interferes with phosphorylation of phosphoproteins in cellular membranes [12], enzyme activities [11,13], and photosynthetic pigments synthesis and other metabolic process [14,15]. The present review, critically discussed the toxic effects on respiration and photosynthesis of agricultural crops and trees of exposure to fluoride.
Basics of Fluoride
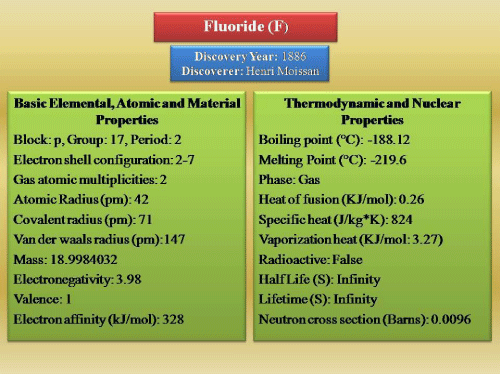
Fluoride is the 13th most abundant element and distributed widely throughout the earth [16]. F is a pale yellow colored gas. It has the atomic number of 9 and atomic weight of 18.9984 at standard temperature and pressure. Fluorine is classified as a halogen, present in the Group VII A of the Periodic Table of Elements (Figure 1; source 17). The halogens consist of fluorine, chlorine, bromine, iodine, and astatine. These all are electromotive elements. They have the nature to exist in the free state as diatomic molecules. Having been electromotivity, these can react with less electromotive elements or chemical groups. Fluoride compounds are formed when the element fluoride combines with other chemical elements. It does not occur in a free state in nature [18]. Assumption on the biochemical properties of fluorides might be similar to those of other halogenated compounds. This assumption may be partially correct. Like other halogen member, inorganic fluorides dissociate in aqueous solution and release to monovalent fluoride anion (F-), along with its associated cation. Fluoride however has many unique chemical properties. These properties had a great impact on the special biochemical physiological properties to the fluorides. For these reason, F can affect the metabolism and mechanisms of action within the living system [18]. In addition to the chemical properties and isotopic nature of fluorine has had an important impact on our understanding of the metabolism, toxicity, and therapeutic effects of fluoride. 19-F is one of the isotopes of F and occurs naturally. This isotope has an extremely short half-life [19,20].
Fluoride Source, Uptake, Translocation and Accumulation by Plants
At higher level of F, it has the great phototoxic effects on vegetation. It released into the environment from a number of industrial sources, use of phosphate fertilizers in agriculture, and weathering of volcanic ashes etc. [21,22]. Some type of soils has high levels of natural fluorides. It could be transferred from soil to roots, and then to above ground parts, or absorbed by leaves from the air. Toxic level of F are found in tree leaves [23,24]. Uptake of F by roots is made through the mechanism like passive diffusion process. Most of the absorbed F is remains exchangeable and readily extractable from the root by mild washing procedures [25,26]. Experimentally, it was found that, mainly F found in the apoplast and some times, little amount also found in the plasmalemma or tonoplast. Levels of F are found low in the shoot as the endodermis acts as an effective barrier. Fluoride reaches the vascular system by a non-selective route that bypasses the endodermis [27]. Uptake of F from air is higher than from the soil. It was found that the level of fluoride in roots is lower than the leaves [28,29]. According to the result of Sloof et al. [30], it was found that the main route of uptake of F by plants is from aerial deposition on the plant surface. From all these studies it could be assumed the pathways of fluoride absorption in the plants. Some experimental works has been highlighted on the F accumulation capacity of plants. Plant uptake and accumulation capacity mainly depends on the plant’s species and the ionic strength of the surrounding environment where it is growing. If the environment has the high concentration of F, then simultaneously F concentration will be higher [10,31,32]. Type of soil has the influence on the F accumulation in plants. Higher concentrations of Ca in soil inhibit the F accumulation in plants from soil [33].
Effects of fluoride on photosynthesis
Multiple metabolic pathways and mechanisms, and consequently, the productivity of agricultural crops might be impairing through the several air pollutants [34]. The response of plants to pollution depends on the toxicity of the chemical element, duration of plant exposure and the species sensitivity [35].
Among the pollutants, F− stands out because of its electromotivity, electronegativity, and high phytotoxic potential. Above all these factors,it has the capacity to preferentially enter through the stomata [36]. F accumulation caused the leaves ultrastructural and structural damages occur in cells and tissues, respectively. After the impairment of cells and tissues, it will be very drastic effects on stomatal conductance and gas exchange of plants [37].
One experiment conducted by Mesquita et al. [38], reported that damage to the epidermis and stomata of leaves from young plants of coffee and orange when these are exposed to hydrogen fluoride (HF) in a semi-open mist chamber. It has the possibility of the loss of regulation in mechanisms of stomatal aperture and closure. In the chloroplasts, F affects enzymes, such as ATP synthase, ribulose bisphosphate carboxylase-oxygenase and sucrose synthase, which have their activity reduced [39].
Fluoride accumulation also hampered the photosynthesis. F affects photosynthesis is mainly by reducing the synthesis of chlorophyll, degradation of chloroplasts, and inhibition of Hills reaction. The chlorophyll content is also decreased and the photosynthetic system of plants is impaired. Ultimately, these caused to decrease the CO2 assimilation and production [40,41].
In plants thylakoid membranes, photosynthetic electron transport chain has been studied after the F exposure. It was found that, accumulation of F inhibit the photosystem-II (PS-II) electron transport rate followed by a subsequent increase in the photosystem-I (PS-I) electron transport rate. This result indicated that state transitions being a mechanism for F toxicity. According to the study of Ballantyne [42], it was reported that F treatment with 190 ppm on plants reduces the photosynthetic pigments. It was also found, in the study of Reddy and Kaur [43].
Plants grown on F contaminated soil has the reduced in photosynthetic capacity, chlorophyll-a (Chl-a) and chlorophyll-b (Chl-b) concentrations, total chlorophyll, carotenoids, and leaf area [44, 45]. The reduction of chlorophyll contents in the plants may be due to F reduced the chlorophyll biosynthesis [46]. Probably, quantity and activity chlorophyll degrading enzyme chlorophyllase goes to higher after the F accumulation [45]. At the semi-arid region, where plants grow on F contaminated soil showed the same effects [47].
Effects of fluoride on respiration
The hazards of fluoride to plant tissues are widely recognized, so injury of vegetation in certain industrialized areas has been attributed to fluoride accumulation [48]. One of the manifestations of fluoride accumulation is alteration of respiration rates [49,50]. Either inhibition or stimulation may occur depending on a number of factors such as the species and age of plant, the length of exposure, and the fluoride concentration. Many investigators have reported fluoride inhibition of respiration in a number of plant species [51]. Others have found that lower concentrations may result in a stimulation of respiration [51].
It was demonstrated by Yu and Miller [50] that fluoride treatment of soybean leaf tissue at both high and low concentrations resulted in an initial stimulation followed by inhibition. Decrease of tissue respiration by fluoride is probably in large part due to inhibition of respiratory enzymes. For example, succinic, malic, and NADH dehydrogenases; enolase; phosphoglucomutase, hexokinase and ascorbic acid oxidase; and ATPase are all known which are inhibited by fluoride except ATPase [52-55].
Inhibition of phosphoglucomutase, the enzyme that participating in sucrose biosynthesis, in higher plants could account for the inhibition of sucrose synthesis in F-fumigated plants [56]. In higher plants, energy metabolic pathways also stunned due to the toxic effects of F. It was found that in ATP forming organelles like chloroplasts, mitochondria, and plasma membrane, ATP synthase enzymes was inhibited by accumulated F. However, at the time of environmental stress condition, plasma membrane associated ATPase (P-ATPase) and tonoplast associated ATPase (V-ATPase) are the main enzymes those has shown the first alteration of their structure. F accumulation also hampered their structure as well as function [57]. From this incident it was demonstrated that these two enzymes are the main initial defensive enzymes against the F injury. On the other hand, Enolase was found to be the most important enzyme of carbohydrate metabolism inhibited by F. Fluoride competition with Mg+2 resulted in a slow decrease of enzyme activity and subsequently, in a complete loss of enzyme activity. In one of the recent findings on six planktonic algae, enolase was inhibited by F [58,59]. The reasons for respiratory stimulation or maintenance of high respiratory rates with fluoride-treated plant tissues are less obvious.
Ross, et al. [60], demonstrated that fluoride treatment on plants resulted in increased use of the pentose phosphate pathway. This was evident with fluoride-stimulated respiration and fluoride-inhibited respiration. The increased use of the pentose phosphate pathway may have been due to inhibition of the glycolytic enzyme enolase.
A possible explanation is that fluoride may be affecting the mitochondrial membrane, as mitochondrial ATPase activity is believed to be accelerated by destruction of membrane integrity [55]. Some other studies [63,64] have suggested that swelling of mitochondria may be related to loss of membrane integrity. It was found that, the membrane is the main site of fluoride action in plants [65,66]. The observed increase in extractable mitochondria may be responsible for part of the fluoride stimulation of tissue respiration as was suggested for pathogen-enhanced respiration in sweet potato tissue [67]. It appears that fluoride treatment induces a number of physiological and biochemical changes in plant tissue that may contribute to increased tissue respiration. The observations of Lee, et al. [54], also stated that glucose-6-phosphate dehydrogenase, catalase, peroxidase, and cytochrome oxidase activities are increased with fluoride treatment. They demonstrated that this phenomenon happened due to the general F injury, and might be exception of glucose-6-phosphate dehydrogenase activity.
Conclusion
In this overview, we reviewed on showing he adverse effects of fluoride compounds on the cellular function of plant biological systems. Several studies demonstrated that fluoride can interact with the cellular processes of photosynthesis and respiration. Although many enzymes involved in these alterations of the photosynthesis and cellular respiration machinery have been identified, however many of the targets and the exact mechanisms/pathways taking part in these events are still unknown. The complexity of fluoride’s effects on these processes are closely related to it’s dose and concentration. However, in the environment, fluoride is frequently remains with other elements in different forms, which does not necessarily lead to more pronounced toxicity. In some cases, antagonistic effects have been reported. Hence, due to the data scarcity on toxic effects of fluoride in combination with other metalloids or metals on the photosynthesis and respiration, it has the necessary to conduct such studies in the field level as well as molecular level also.
- Jentsch TJ, Stein V, Weinreich F, Zdebik AA (2002) Molecular structure and physiological function of chloride channels. Physiol Rev 82: 503–568. Link: https://goo.gl/UKvX1i
- Edwards JC, Kahl CR (2010) Chloride channels of intracellular membranes. FEBS Lett 584: 2102–2111. Link: https://goo.gl/2JU2YK
- Zimmermann MB (2011) The role of iodine in human growth and development. Semin Cell Dev Biol 22: 645–652. Link: https://goo.gl/B5wKDq
- Jagtap S, Yenkie MK, Labhsetwar N, Rayalu S (2012) Fluoride in drinking water and defluoridation of water. Chem Rev 112: 2454–2466. Link: https://goo.gl/gsjylk
- WHO (1984) Fluorine and Fluorides, Environmental Health Criteria 36, IPCS International Programme on Chemical Safety (World Health Organization, Geneva). Link: https://goo.gl/VtqO0Z
- Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7: 60-72. Link: https://goo.gl/DLzKvu
- Kanduti D, Sterbenk P, Artnik B (2016) Fluoride: a review of use and effects on health. Mater Sociomed 28: 133-137. Link: https://goo.gl/Ooi4xI
- WHO (2006) Fluoride in Drinking Water (World Health Organization, Geneva). Link: https://goo.gl/U3voyZ
- Dando N, Xu W, Peace JN (2008) Continuous measurement of peak hydrogen fluoride exposures in aluminum smelter potrooms: instrument development and in-plant evaluation. J Occup Environ Hyg 5: 67-74. Link: https://goo.gl/Og9uz0
- Kebede A, Retta N, Abuye C, Whiting SJ, Kassaw M, et al. (2016) Dietary Fluoride Intake and Associated Skeletal and Dental Fluorosis in School Age Children in Rural Ethiopian Rift Valley. Kruger M, Weiler H, eds. Int J Environ Res Public Health 13: 756. Link: https://goo.gl/EcCRQN
- Waugh DT, Potter W, Limeback H, Godfrey M (2016) Risk Assessment of Fluoride Intake from Tea in the Republic of Ireland and its Implications for Public Health and Water Fluoridation. Tchounwou PB, ed. Intl J Environ Res Public Health 13: 259. Link: https://goo.gl/yPSdNG
- Chang SC, Kaufman PB (2000) Effects of staurosporine, okadaic acid and sodium fluoride on protein phosphorylation in gravi-responding oat shoot pulvini. Plant Physiol Biochem 38: 315–323. Link: https://goo.gl/bma6xY
- Westram A, Lloyd JR, Roessner U, Riesmeier JW, Kossmann J (2002) Increases of 3-phosphoglyceric acid in potato plants through antisense reduction of cytoplasmic phosphoglycerate mutase impairs photosynthesis and growth, but does not increase starch contents. Plant, Cell Env 25: 1133–1143. Link: https://goo.gl/taa3rU
- Baunthiyal M, Ranghar S (2014) Physiological and biochemical responses of plants under fluoride stress: an overview. Fluoride 47: 287–293. Link: https://goo.gl/TNDTRp
- Kamaluddin M, Zwiazek JJ (2003) Fluoride inhibits root water transport and affects leaf expansion and gas exchange in aspen (Populus tremuloides) seedlings. Physiologia Plantarum 117: 368–375. Link: https://goo.gl/lkDRB1
- Jordan RA, Markovic L, Gaengler P (2008) Fluoride availability from natural resources in The Gambia--implications for oral health care. Int Dent J 58: 237-242. Link: https://goo.gl/JEmvq0
- Bharti VK, Giri A, Kumar K (2017) Fluoride Sources, Toxicity and Its Amelioration: A Review. Peertechz J Environl Sci Toxicol 2: 021-032. Link: https://goo.gl/D4bA3l
- Kurdi MS (2016) Chronic fluorosis: The disease and its anaesthetic implications. Indian J Anaesth 60: 157-162. Link: https://goo.gl/SeHYSu
- Leech HE, "The Physical and Chemical Properties of Fluorine and the Physical and Chemical Properties and Uses of Hydrogen Fluoride," 1956, Mellor's Comprehensive Treatise on Inorganic and Theoretical Chemistry, London; Longmans, Green, (Suppl II), 1:46-146.
- National Center for Biotechnology Information. PubChem Compound Database; CID=24524, https://pubchem.ncbi.nlm.nih.gov/compound/24524 (accessed Mar. 16, 2017).
- Mackowiak CL, Grossl PR, Bugbee BG (2003) Biogeochemistry of fluoride in a plant-solution system. Journal of Environmental Quality 32: 2230–2237. Link: https://goo.gl/812Tx5
- Cronin SJ, Neall VE, Leconintre JA, Hedley MJ, Loganathan P (2003) Environmental hazards of fluoride in volcanic ash: A case study from Ruapehu volcanic, New Zealand. J Volcanology and Geother Res 121: 271–291. Link: https://goo.gl/fZqyoW
- Ruan J, Wong M (2001) Accumulation of fluoride and aluminium related to different varieties of tea plant. Environ. Geochem Health 23: 53-63. Link: https://goo.gl/WUe9Yb
- Shu W, Zhang Z, Lan C, Wong M (2003) Fluoride and aluminium concentrations of tea plants and tea products from Sichuan Province, PR China. Chemosphere 52: 1475-1482. Link: https://goo.gl/TTVFm5
- Larsen M, Ucisik AS, Trapp S (2005) Uptake, metabolism, accumulation and toxicity of cyanide in Willow trees. Environ Sci Technol 39: 2135-2142. Link: https://goo.gl/RbUhaV
- Garrec JP, Letoureneur L (1983) Fluoride absorption by the root and foliar tissues of horse bean (Vicia faba minor; calciole) and lupine (Lupinus luteus; calcifuge). Fluoride 14: 30-38. Link: https://goo.gl/bkjlvu
- Singh V, Gupta MK, Rajwanshi P, Mishra S, Srivastava S, et al. (1995) Plant uptake of fluoride in irrigation water by ladyfinger (Abelmorchus esculentus). Food Chem Toxicol 33(5): 399-402. Link: https://goo.gl/bXtU5u
- Groth E (1975) Fluoride pollution, along the food chain. Environ, 17: 29–38. Link: https://goo.gl/QB3azs
- Pitman MG (1982) Fluoride: Transport across plant roots. Quart Res Biophysics 15: 481-554. Link: https://goo.gl/Ve36lN
- Sloof W, Eerens H, Janus J, Ros J (1989) Integrated criteria document: Fluorides. Bilthoven, National Institute of Public Health and Environmental Protection (Report No. 758474010). Link: https://goo.gl/IwziFV
- Stevens DP, McLaughlin MJ, Alston AM (1998) Phytotoxicity of the fluoride ion and its uptake from solution culture by Avena sativa and Lycopersicon esculentum. Plant and Soil 200: 119-129. Link: https://goo.gl/JWuuwF
- McCune DC, Hitchcock AE, Jacobson JS, Weinstein LH (1965) Fluoride accumulation and growth of plants exposed to particulate cryolite in the atmosphere. Contrib Boyce Thompson Inst 23: 1-11. Link: https://goo.gl/mISDTV
- Sheldrake RB, Rose WI, Reed MH, Lichte FE, Finnegan DL (1978) Lime and charcoal amendments reduce fluoride absorption cultured in pertitepeat medium. J Amer Soc Hort Sci 103: 268-270. Link: https://goo.gl/Tgb4Cv
- Arndt U, Flores F, Weinstein LH (1995) Fluoride Effects on Plants, Diagnose of Injury in the Vegetation of Brazil. Porto Alegre.
- Oguchi R, Hikosaka K, Hirose T (2005) Leaf Anatomy as a Constraint for Photosynthetic Acclimation: Differential Responses in Leaf Anatomy to Increasing Growth 83 Irradiance among Three Deciduous Trees. Plant Cell and Environ 28: 916-927. Link: https://goo.gl/tKLL2J
- Franzaring J, Klumpp A, Fangmeier A (2007) Active Biomonitoring of Airborne Fluoride near an HF Producing Factory Using Standardized Grass Cultures. Atmospheric Environ 41: 4828- 4840. Link: https://goo.gl/gd9Ec2
- Alves ES, Moura BB, Domingos M (2008) Structural Analysis of Tillandsia usneoides L. Exposed to Air Pollutants in São Paulo City-Brazil. Water Air Soil Pollu 189: 61-68. Link: https://goo.gl/a5XsDC
- Mesquita GL, Tanaka FAO, Cantarella H, Mattos D (2011) Atmospheric Absorption of Fluoride by Cul- tivated Species. Leaf Structural Changes and Plant Growth. Water, Air and Soil Pollu 219: 143-156. Link: https://goo.gl/UVZIZl
- Parry MAJ, Schmidt CNG, Gutteridge S (1984) Inhibition of Ribulose-P2 Carboxylas/Oxigenase by Fluoride. J Experl Botany 35: 161-198. Link: https://goo.gl/4881nh
- Yamauchi M, Choi WK, Yamada Y (1983) Fluoride inhibition of photosynthesis in certain crop plants. Soil Sci Plant Nutr 29: 549-553. Link: https://goo.gl/Py5j2X
- Domingues RR, Mesquita GL, Cantarella H, Mattos D (2011) Suscetibilidade do Capim-Colonião e de Cultivares de Milho ao Flúor. Bragantia 71: 729-736. Link: https://goo.gl/4MekYR
- Ballantyne DJ (1991) Fluoride and photosynthetic capacity of azalea (Rhododendron) cultivars. Fluoride 24: 11-16. Link: https://goo.gl/g2cpcL
- Reddy MP, Kaur M (2008) Sodium fluoride induced growth and metabolic changes in Salicornia brachiata. Roxb. Water Air Soil Pollut 188: 171-179. Link: https://goo.gl/aEUuIf
- Kumar KA, Rao AVB (2008) Physiological responses to fluoride in two cultivars of Mulberry. World J Agric Sci 4: 463-466. Link: https://goo.gl/PoyNUv
- Ram A, Verma P, Gadi BR (2014) Effect of fluoride and salicylic acid on seedling growth and biochemical parameters of watermelon (Citrullus lanatus). Fluoride 47: 49–55. Link: https://goo.gl/ovnRxg
- Gupta S, Banerjee S, Mondal S (2009) Phytotoxicity of fluoride in the germination of paddy (Oryza sativa) and its effect on the physiology and biochemistry of germinated seedlings. Fluoride 42: 142-146. Link: https://goo.gl/hs9TTq
- Baunthiyal M, Sharma V (2014) Response of three semi-arid plant species to fluoride; consequences of chlorophyll Florescence. Int J Phytorema 16: 397-414. Link: https://goo.gl/0s5gZN
- Thomas MD (1961) Effects of air pollution on plants. — In Air Pollution, World Health Organ. Monogr (Geneva) 46: 233-278. Link: https://goo.gl/JfgcE1
- Weinstein LH (1961) Effects of atmospheric fluoride on metabolic constituents of tomato and bean leaves. Contrib. Boyce Thompson Inst 21: 215-231. Link: https://goo.gl/EfnrAU
- Yu MH, Miller GW (1967) Effect of fluoride on the respiration of leaves from higher plants. Plant Cell Physiol 8: 483-493. Link: https://goo.gl/ohQfIQ
- McNulty IB, Lords JB (1960) Possible explanation of fluoride-induced respiration in Chlorella pyrenoidosa. Sci 132: 1553-1554. Link: https://goo.gl/Bre8N8
- Lovelace CJ, Miller GW (1967) In vitro effects of fluoride on tricarboxylic acid cycle dehydrogenases and oxidative phosphorylation: Part I. J Histochem Cytochem 15: 195-201. Link: https://goo.gl/7L2cjk
- Melchior NC, Melchior JB (1956) Inhibition of yeast hexokinase by fluoride ion. Sci 124: 402-403. Link: https://goo.gl/elBX2f
- Lee C, Miller GW, Welkie GW (1965) The effects of hydrogen fluoride and wounding on respiratory enzymes in soybean leaves. Air Water Pollut Int J 10: 169-181. Link: https://goo.gl/EtvnWv
- Miller JE, Miller GW (1974) Effects of fluoride on mitochondrial activity in higher plants. Physiol Plant 32: 115–121. Link: https://goo.gl/A1F0xe
- Yang SF, Miller GW (1963) Biochemical studies on the effect of fluoride on higher plants. Metabolism of carbohydrates, organic acids and amino acids. J Biochem 88: 505-509. Link: https://goo.gl/BTwEq9
- Rakowski KJ (1997) Hydrogen fluoride effects on plasma membrane composition and ATPase activity in needles of white pine (Pinus strobus) seedlings pretreated with 12 h photoperiod. Trees Struct Funct 11: 248-253. Link: https://goo.gl/IPuAWc
- Strunecka A, Patocka J, Blaylock RL, Chinoy NF (2007) Fluoride interaction: from molecule to disease. Curr Signal Transd 2: 190-213. Link: https://goo.gl/yGSNBJ
- Hekman WE, Budd K, Palmer GR, MacArthur DJ (2004) Responses of certain freshwater planktonic algae to fluoride. J Phycol 20: 243-249. Link: https://goo.gl/9pgcHg
- Ross CW, Wiebe HH, Miller GW (1962) Effect of fluoride on glucose catabolism in plant leaves. Plant Physiol 37: 305-309. Link: https://goo.gl/Wpk4lN
- Lee C, Miller GW, Welkie GW (1965) The effects of hydrogen fluoride and wounding on respiratory enzymes in soybean leaves. Air Water Pollut Int J 10: 169-181. Link: https://goo.gl/EtvnWv
- Klingenberg M, Schollmeyer P (1961) Redox reactions in mitochondria under the control of ATP. Proc. Tnt. Congr. Biochem. 5th, Moscow, 5: 46-68. Link: https://goo.gl/OQzuz6
- Earnshaw MJ, Truelove B (1968) Swelling and contraction of Phaseolus hypocotyl mitochondria. Plant Physiol 43: 121-129. Link: https://goo.gl/aQuRj8
- Lee TT (1968) Effect of ozone on swelling of tobacco mitochondria. Plant Physiol 43: 133-139. Link: https://goo.gl/nYGgkV
- Ramagopal S, Welkie GW, Miller GW (1969) Fluoride injury of wheat roots and calcium nutrition. Plant Cell Physiol 10: 675-685. Link: https://goo.gl/nGk2tp
- Wei LL, Miller GW (1972) Effect of HF on the fine structure of mesophyll cells from Glycine max, Merr. Fluoride 5: 67-73. Link: https://goo.gl/ouy7ry
- Asahi T, Honda Y, Uritani I (1966) Increase of Mitochondrial Fraction in Sweet Potato Root Tissue after Wounding or Infection with Ceratocystis fimbriata. Plant Physiol 41: 1179-1184. Link: https://goo.gl/TtPWx9
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
