Archives of Depression and Anxiety
Antidepressants and memory effects of ketamine under the neuromolecular view: A literature review
Da Silva GP Felipe1*, Rezende M Gabriel2, Lohana Pompelli Scapatici1, Luísa Zanelatto de Araujo1, Bruna Carrara Lombardi1, Caroline Vidal1, David Batista Wiedmer1, Lucas Schoeler1, Minhoto R Gisele1 and Andreatini Roberto3
2Department of Medicine, Federal University of Paraná, UFPR, Brazil
3Department of Pharmacology, Biological Sciences Sector, Federal University of Paraná, Center Polytechnic, UFPR, Brazil
Cite this as
Da Silva GPF, Gabriel RM, Scapatici LPS, de Araujo LZ, Lombardi BC, et al. (2023) Antidepressants and memory effects of ketamine under the neuromolecular view: A literature review. Arch Depress Anxiety 9(1): 005-016. DOI: 10.17352/2455-5460.000073Copyright
© 2023 Da Silva GPF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Objective: Major Depressive Disorder (MDD) has as diagnostics characteristics chronic deep sadness, anhedonia, sleeping disorder, lower energy, and cognition impairment like memory deficits. Among the pharmacological treatments that have been used until the moment, most of them act by monoaminergic pathways. Overall, the antidepressant effects promoted by this kind of medication usually delay starting, resulting in treatment resistance by the patients; moreover, in some cases, this kind of treatment has shown to be inefficient in depression remission. With this, new treatments have been studied for resistant cases and an immediate antidepressant effect, for example, ketamine – whose action occurs in glutamatergic pathways. This study aimed to analyze, from a literature review, the molecular mechanisms involved in the action of ketamine - focusing on the neuroplastic hypothesis of depression.
Methods: A literature search was conducted in PubMed, MEDLINE, and SciELO databases using the following terms as descriptors: "ketamine AND depression AND Neuroplasticity," with criterion PICO, resulting in 60 bibliographic texts.
Results/discussion: The studies analyzed demonstrated that ketamine could exert its antidepressant effects through the inhibition of GABAergic interneurons, activation of TRK-B/AKT/mTORC pathways involved with cell survival/growth through the neurotrophine BDNF and increased activation of AMPAr by glutamate. Furthermore, it is evident that the pharmacodynamics of ketamine involves different molecular cascades present in the impaired neural plasticity pathways in individuals with MDD.
Conclusion: Thus, more research on the effectiveness of ketamine is needed to consolidate its use in MDD and to evolve with glutamatergic pharmacological therapy for other mental disorders, such as bipolar and neurodegenerative affective disorders, an example of Alzheimer's disease.
Introduction
It can be said that depression is a disorder based on symptoms that form a syndrome, causing functional impairment [1]. According to the Diagnostic and Statistical Manual of Mental Disorders in its fifth edition (DSM-5), Major Depressive Disorder (MDD) diagnostic characteristics are the presence of deep chronic sadness, anhedonia, sleep disorders, decreased energy, and cognitive problems, such as memory impairment.
Depressive disorders affect approximately 16% of the world's population, resulting in social, physiological, and economic losses [2,3]. The 12-month prevalence of major depression varies between countries, but it can be around 6%. However, the lifetime risk of depression varies between 15% and 18% [1]. When assessing the prevalence of depression or depressive symptoms in outpatients, it is 27%, ranging from 17% to 53% in the different medical specialties [4]. Depression throughout life is twice as common in women as in men [1].
The first episode of MDD can occur at any age, but in general, it begins in the late teens to mid-40s, with almost 40% of individuals having their first episode of depression before the age of 20. However, MDD can also occur late, affecting the elderly [1,3].
MDD is quite variable, with some individuals not achieving remission and others having a chronic and recurrent course. In the vast majority of patients, the pathology is characterized by depressive episodes of unpredictable frequency and duration, alternating with moments of well-being for the patient [1,3].
So far, there is no single factor or mechanism capable of explaining the entire pathophysiology of major depression. Different causes or pathophysiological factors may be involved in episodes of different patients or even in different episodes in the same patient. Thus, at each episode, it is always necessary to evaluate the biological and psychosocial factors [1].
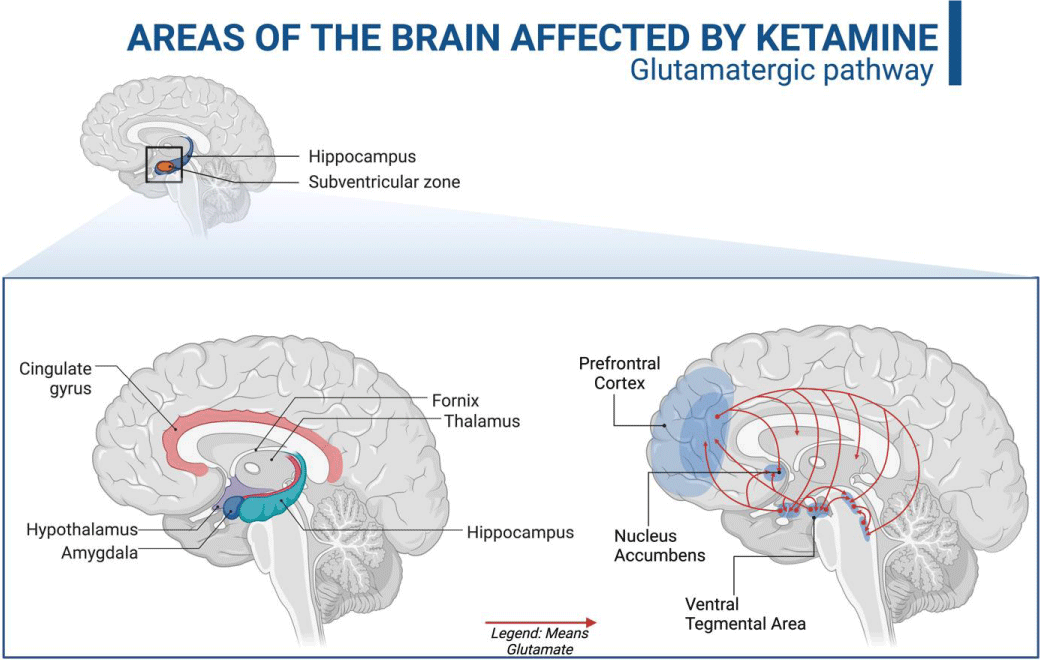
Studies involving neuroimaging and post-mortem analysis of the brain of individuals affected by MDD have enabled a broader understanding of this pathology. Through these studies, it can be elucidated that the reduction in the volume of brain areas such as the prefrontal cortex, the nucleus accumbens, and the hippocampus originates from the dysfunction of brain neuroplastic activity [4].
Located at the base of the midbrain, the ventral tegmental area (VTA) is the base of the mesolimbic and mesocortical circuits. VTA is related to the motivation and reward circuit due to important dopaminergic projections directed to the prefrontal cortex, the nucleus accumbens, and the hippocampus [5,6]. Its activity is regulated by glutamatergic (excitatory) and GABAergic (inhibitory) afferents by a pacemaker mechanism - which is compromised in MDD [7,8].
Another prominent factor in patients with MDD is the significant loss of synapses and neuronal populations in regions of the prefrontal cortex and the basal nucleus, such as the nucleus accumbens. These alterations are closely related to impairments in the pleasure and reward mechanism, as well as in the processing of emotions and the function of cognitive processes [9].
Brain changes in the hippocampal region associated with MDD are consistent with the idea that a prolonged depressive episode can lead to hippocampus atrophy, impairing explicit or declarative memory fixation [4,10,11].
We also have those neurodegenerative diseases such as Parkinson's Disease (PD) that are involved with neural loss movements by an accumulation of Lewis bodies in dopaminergic regions that are extremely important for memory, the same regions that ketamine can act. A study with a rodent model of Parkinson's disease concluded that after using ketamine with imipramine, ketamine reversed depressive-like behaviors and short-term memory impairment in rats with bilateral CNS lesions, indicating a good profile for its use in PD patients [2].
The traditional pharmacological treatments used in MDD act mainly on monoamines - serotonin, noradrenaline, and dopamine. The search for other treatment options is due to the delay in the onset of therapeutic results and the side effects of conventional antidepressants, which leads to patients giving up.
In addition to these factors, it is essential to note that many patients do not reach or remain in remission, and approximately one-third do not respond to treatment with two or more first-line antidepressants. Thus, drugs that allow a rapid antidepressant action and that also act in the so-called treatment-resistant depression have been studied, such as ketamine [1,12].
Ketamine is a derivative of phencyclidine hydrochloride (phencyclidine hydrochloride – PCP), synthesized by Stevens in 1965, and has its anesthetic action as the main pharmacological indication in humans and animals. In recent years, its action as an antidepressant drug has been investigated. It is found as a racemic mixture, thus possessing properties of optical isomerism, and its enantiomers are R-(-)
-ketamine (arketamine) and S-(+)-ketamine (esketamine) [1,13]. Recently, esketamine has been approved by the U.S. Food and Drug Administration (FDA) as a nasal spray for use in patients with refractory depressive disorder [13].
Ketamine can be used as an antidepressant but is also like a drug of abuse. For being of the class of dissociative drugs, it can promote an effect of desire, euphoric rush, sensory distortions and mild hallucinations, but also can produce bad sensations as they call a “keyhole” – a dissociative state that the person lost the sense of time and space, also affecting the equilibrium [14-17].
The abusive use of ketamine started in the 70th but turned popular in 2000’ year. The United States Surgeon General Report on Alcohol, Drugs, and Health notes the lifetime of use in life in 1,1% (approximately 3 million persons) and a medium start age of 19,6 years old. Monitoring the Future has shown that the prevalence of the use of ketamine in the last year was between 0.7% and 1.2% in the USA. A study of the epidemiologic use of ketamine in Europe showed that the United Kingdom has an extensive use, having a prevalence of 70% over life in those people who frequented clubs and dance clubs, being considered the country with the most use of ketamine [18,19].
In the last 20 years, the number of chronic addictions to ketamine has grown out of Europe; today, one of the most addictive continents in ketamine is Asia. The 2019 World Drug Report by the United Nations Office on Drugs Control classified the use of ketamine as a “new psychoactive substance (NPS) that could pose a threat to public health and not under the control of international drug conventions” [20,21].
As said before, ketamine consists of a racemic mixture consisting of two optical enantiomers: (R)-ketamine (arketamine) and (S)-ketamine (esketamine). It is already known that the racemic mixture can cause addiction. However, between the two enantiomers, new studies suggested that (S)-ketamine (esketamine) has more abuse potential than the other one because it can cause more dissociative symptoms.
When it was analyzed a study that used CPP (Conditional Pavlovian Paradigma) in rats with an application of injection in equal doses of (S)-ketamine and (R)-ketamine, it was possible to observe CPP and locomotor sensibility in the rat with (S)-ketamine different from his enantiomer, that did not show CPP or even locomotor sensibility (that is a symptom searched by the addicts when used ketamine) [22,23].
In the last 20 years, it was reused the substance ketamine for a lot of psychiatric disorders, such as MDD, but also others, such as bipolar disorder (BD) [24], alcoholism treatment [25], generalized anxiety disorder, and post-traumatic stress disorder (PTSD)(54). The most commonly and conciliated studies today involve MDD and treatment-resistant depression (TRD), which have a good and fast improvement in the symptoms, as we can see in a recent study that evaluated 41 patients with treatment-resistant depression completed a single-site randomized, double-blind crossover comparison of single infusions of ketamine and midazolam (an active placebo control) as a result from the study, was found that fifty-nine percent of participants met response criteria after repeated infusions, with a median of three infusions required before achieving response. Participants had no further change in Montgomery-Åsberg Depression Rating Scale (MADRS) scores during weekly maintenance infusions [26].
Many studies presented that infusion of subanesthetic doses of ketamine (0.5 -1.0 mg/kg IV of 40 min) produces antidepressants and anti-suicide symptoms quickly in individuals with MDD, TRD, and BD [27,28]. Similarly, some results were found with IN applications of esketamine (Spravato ®) that the FDA approved. Now it is having pilot studies using arketamine IV for TRD, having already a phase 2 study in development trying to prove the efficiency of arketamine to reduce antidepressive symptoms in an individual with TRD [24,25,28,29].
Ketamine's mechanism of action is complex, as it acts on several neurotransmitters in numerous binding sites, including glutamate, opioid, and GABAergic receptors, and may also act indirectly on monoamines (serotonin, noradrenaline, and dopamine). The glutamatergic system is, nevertheless, where the main action of ketamine occurs, which acts in these circuits as a non-competitive antagonist of NMDA (N-methyl-D-aspartate) receptors [1]. Glutamate is the primary excitatory neurotransmitter of the Central Nervous System (CNS). It plays an essential role in the mechanisms underlying synaptic plasticity, which are the physiological basis of cognitive and memory processes [30,31].
Glutamate acts on the CNS by binding to postsynaptic glutamatergic receptors, classified as ionotropic (iGluRs – ionotropic glutamate receptors) and metabotropic (mGluRs – metabotropic glutamate receptors). Activation of mGlurRs triggers slow postsynaptic responses, either excitatory or inhibitory, from G protein signaling, inducing the opening of Na+, K+ channels.
The iGluRs response, triggered by glutamatergic signaling, is more present and is, therefore, the most studied. Such receptors are tetrameric proteins that are arranged around the central pore and can be of three types: NMDA (N-methyl-D-aspartate), AMPA (amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid), and KA (kainate) [12,30–32]. AMPA receptors are primarily responsible for postsynaptic neuron depolarization, activated upon binding to glutamate in the synaptic cleft. AMPAr, when activated, triggers postsynaptic responses [12,30-32].
NMDA receptors form a voltage-gated ion channel, having a pore blocked by Mg2+. For its activation, a pre-depolarization of the intracellular space is necessary concomitantly with the binding of glutamatergic agonists to the receptor binding site. This pre-depolarization can be caused by EPSP (excitatory postsynaptic potential) generated by AMPA receptors [12,30,32]
Due to their role in learning and memory processes, NMDA receptors are important, exercising the main focus of the studies on iGluRs-type receptors. The NMDA receptors present greater permeability to Ca2+ than the AMPA and KA receptors, acting in the long-term potential (LTP - long-term potential) and long-term depression (LTD - long-term depression), allowing, therefore, for Neuroplasticity to occur [1,12,30,32,33].
“Neuroplasticity can be defined as an adaptive change in the structure and functions of the nervous system, which occurs at any stage of ontogeny, as a function of interactions with the internal or external environment, or even as a result of injuries, traumas or injuries itself. That affects the neural environment” (Phelps, 1990) [34].
Research involving neural plasticity falls into three general categories: Metabolic: comprising metabolic changes in cortical and subcortical areas; Neurochemical: which analyzes the functional changes in synapses, investigating mechanisms that increase the synthesis/release of neurotransmitters or the potentiation of postsynaptic responses, as a result of stimulating situations, learning or injuries and morphological: which characterize changes in the structure of synapses and neurons, such as the regeneration and branching of axons, increase in the size of cell bodies, the number of dendrites, neurons and also the density of receptors [35].
Studies using imaging techniques in post-mortem histological samples of depressed individuals or animal models of induced stress allowed the observation that brain regions usually affected by depressive disorders - such as the amygdala, hippocampus, prefrontal cortex, VTA, and nucleus accumbens – are areas that have marked neuroplastic activity. Based on these findings, analyzing MDD using a different approach than the monoaminergic approach was possible, creating the neuroplastic hypothesis of depression [4,32]
As previously mentioned, ketamine acts mainly on glutamatergic pathways, which are fundamental for activating the phenomenon of Neuroplasticity. Therefore, it is assumed that ketamine has direct and indirect effects on the regulation of neural plasticity and that through these effects, it can exert an antidepressant action [1,3,4,32].
This work aimed to analyze, based on a literature review, the mode of action of ketamine on the different regions of the brain affected by MDD and what are the molecular mechanisms involved – focusing on the neuroplastic hypothesis of depression.
Methodology
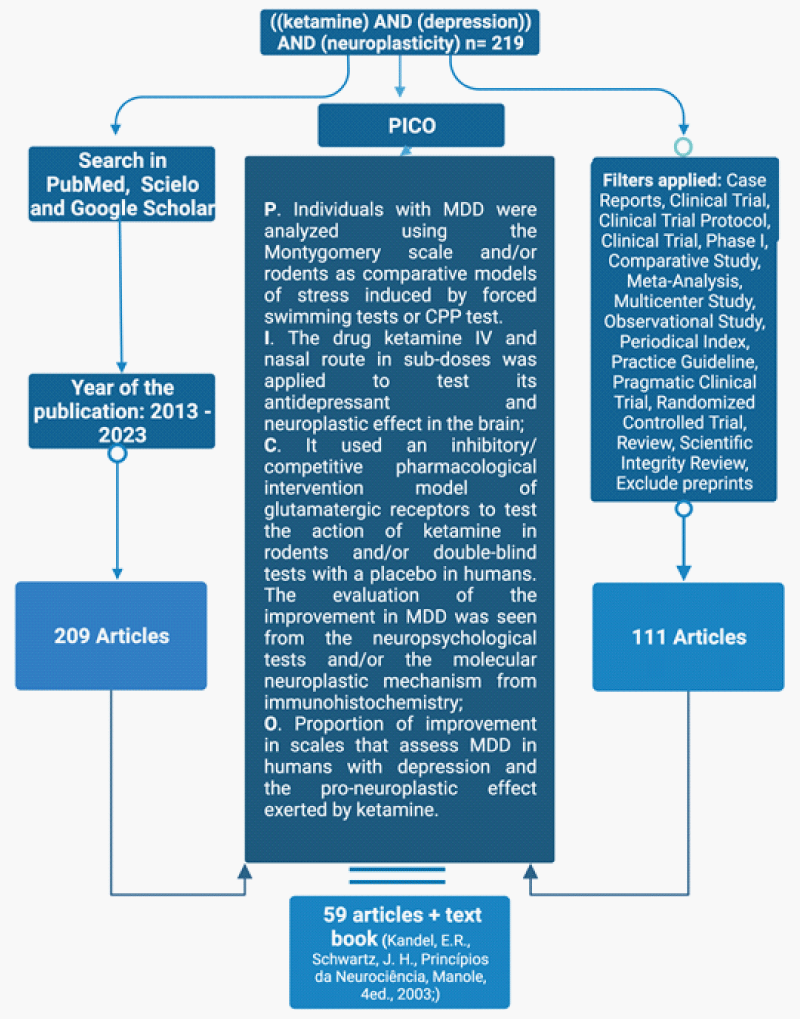
The bibliographic survey was carried out in the PubMed, Scielo, and Google Scholar databases using the following terms as descriptors: “ketamine AND depression AND Neuroplasticity.” The search for descriptors in the databases was carried out on December 12, 2022, and February 2, 2023, searching for theses and articles published between 2013 and 2023, which resulted in 219 articles/theses. Among these, the first criterion to analyze the publication was the date, excluding those that did not cover the period from 2013 to 2023. From this, 111 articles/theses were selected according to the types of studies carried out. As a result, their abstracts were read, following the established PICO’s criteria, resulting in 59 articles/theses for review plus the book Principles of Neuroscience (Manole, 4th ed., 2003; Kandel, E.R., Schwartz, J. H (Figure 1).
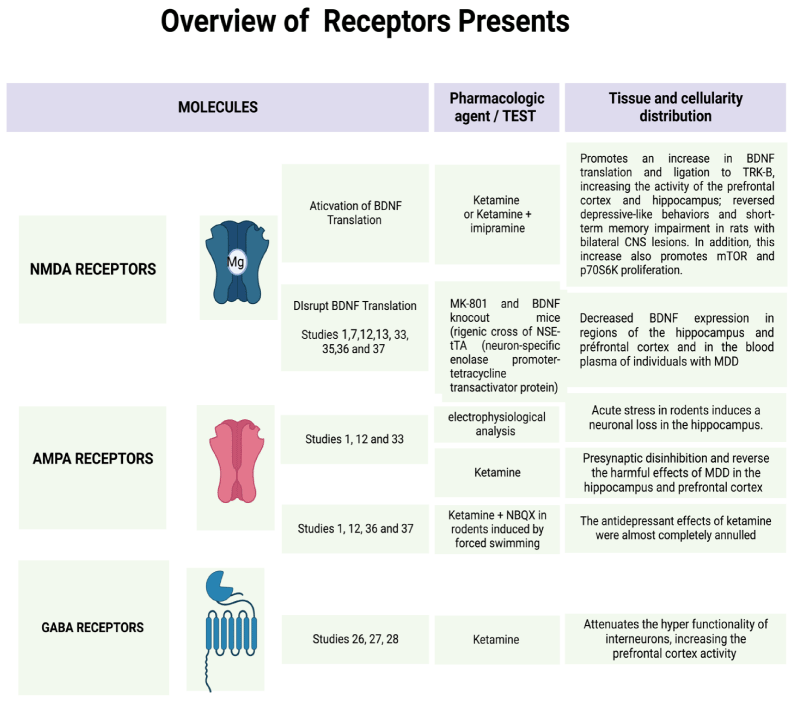
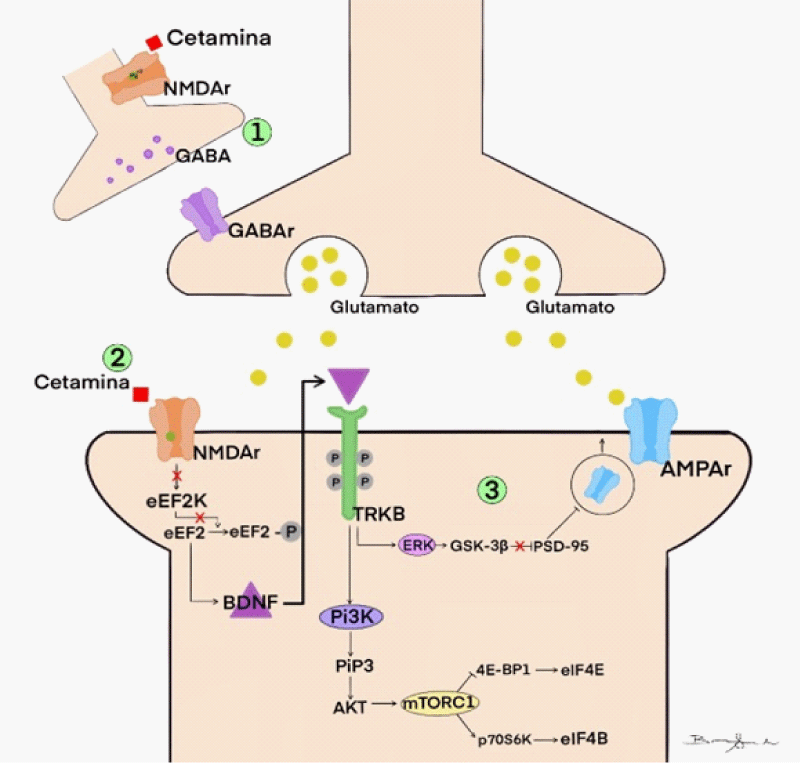
Furthermore, also it was created the Figure 2 explaining the functionality of the receptors and their action with or without ketamine and toward some pharmacies that can block them (Figure 2).
Results and discussion
Ketamine inactivates NMDAr
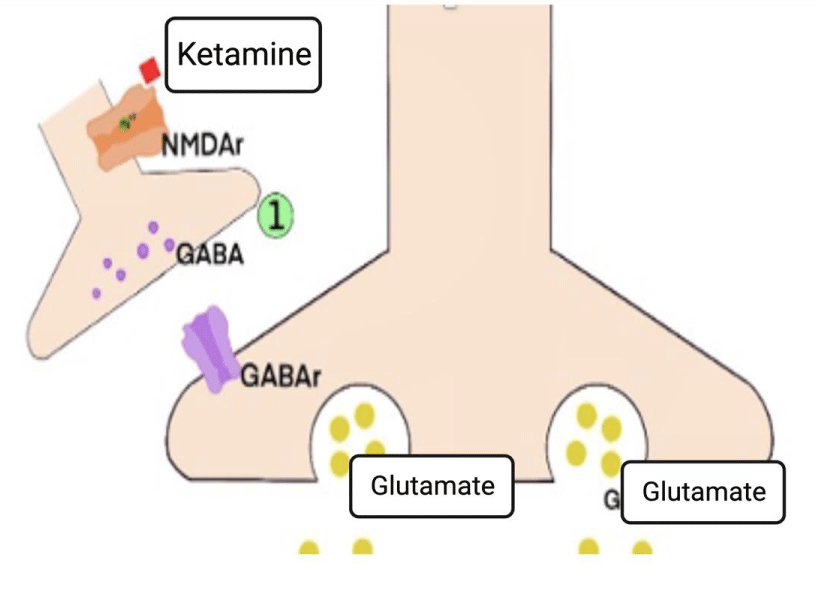
Ketamine's antidepressant effects are related to its non-competitive antagonistic action on ionotropic NMDA glutamatergic receptors [1,11]. These receptors are heterogeneously distributed throughout the brain, being in more significant numbers in the regions of the prefrontal cortex, hippocampus, amygdala, nucleus accumbens, and ventral tegmental area, these being essential centers of memory, cognition, and emotions [1,31] (Figure 3).
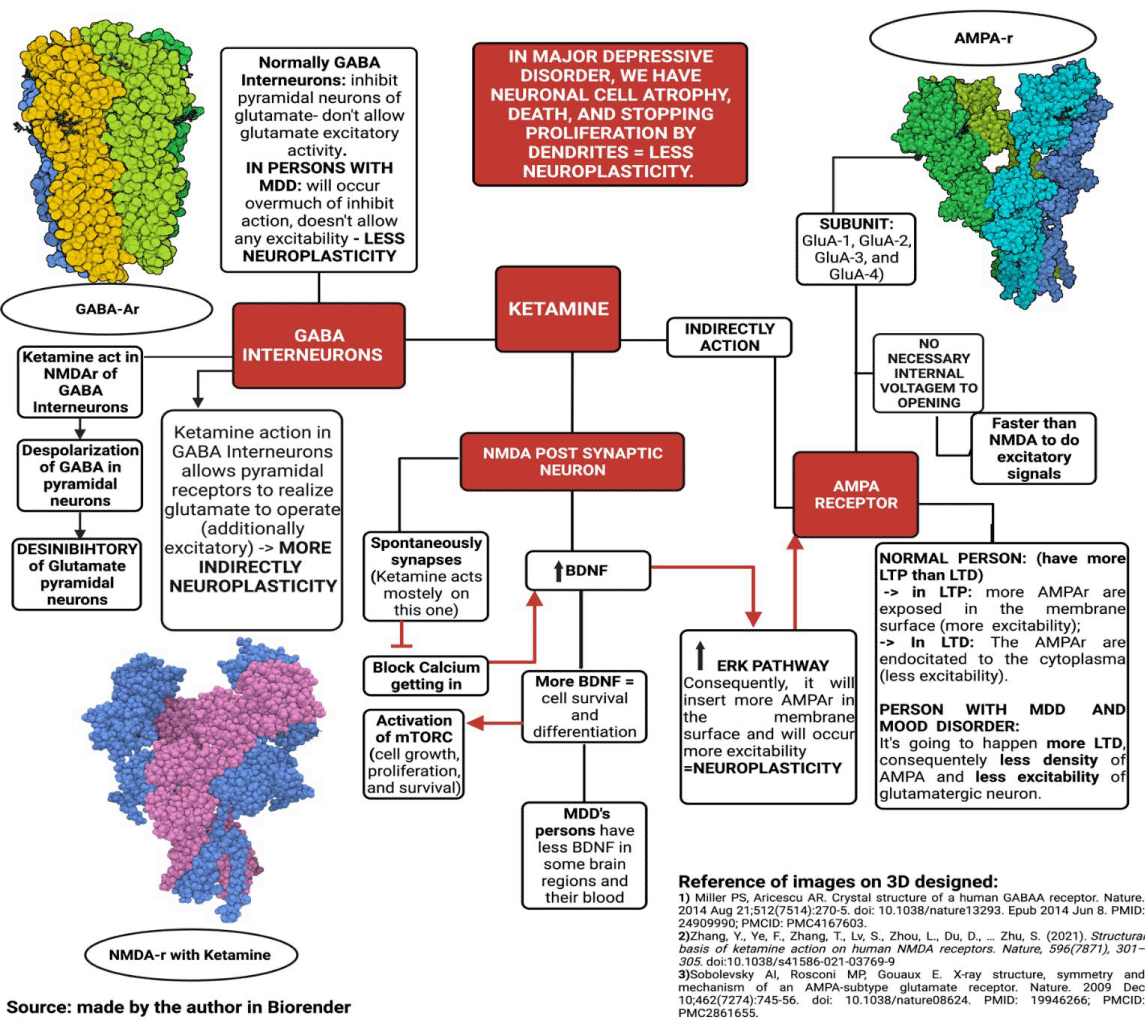
The rapid antidepressant action induced by ketamine is due to the inhibition of NMDA receptors present in GABAergic interneurons - whose function is to cause hyperpolarization in pyramidal neurons in cortical and subcortical regions - as well as the postsynaptic NMDA receptors of glutamatergic synapses excitations mediated by AMPA receptors [1,11,12,36].
Thus, ketamine controls the excitability of neurons; however, the drug also acts by directly regulating synaptic plasticity. This effect occurs because when ketamine binds to NMDA receptors, it triggers a series of intracellular reactions that lead to the translation of neurotrophic factors important for controlling cell growth and development. This mechanism seems to play a central role in the antidepressant effects of the drug [11,12] (Figure 4).
1) The disinhibitory action of ketamine in GABAergic interneurons
Gamma-Aminobutyric Acid (GABA) is the primary inhibitory neurotransmitter in the central nervous system of mammals. Inhibitory associative neurons, or simply interneurons, use GABA as their primary neurotransmitter, modulating emotional, motor, and higher psychic excitatory circuits - acting through cerebral loops. This regulatory mechanism occurs due to the mapped activity of this neuronal population, which, when activated in a brain area, triggers an attenuating response in the corresponding area - by binding to a postsynaptic GABAergic receptor [32].
The inhibitory effects of GABA result from its action on two distinct classes of receptors: ionotropic (type A) and metabotropic (type B). The ionotropic type A GABA receptors are ligand-dependent Cl- ion channels that act quickly, generating neuronal hyperpolarization. Metabotropic B-type GABA receptors produce a slow but prolonged response via G protein and second messenger. Changes in the activity of GABAergic receptors are associated with the occurrence of neurological and psychiatric conditions such as epilepsy, depression, sleep disorders, and cognitive deficits [37].
GABAB receptors are present in the presynaptic membrane of pyramidal neurons, and their activation triggers a signaling cascade that alters the permeability of K+ and Ca2+ ions by decreasing cAMP production. This mechanism generates PEPS modulation by inhibiting the release of glutamate in the synaptic cleft, thus modulating the glutamatergic pathways [38,39]. In individuals with MDD, these mechanisms of dysfunctionalities help to explain the occurrence of symptoms such as anhedonia, cognitive deficits, and memory impairment. This effect occurs due to the hyper functionality of GABAB receptors, which causes excessive inhibition of glutamatergic neurons (Figure 5.1) [40].
Studies suggest that the application of ketamine in sub-anesthetic doses attenuates this hyper functionality, resulting in an increase in the prefrontal cortex activity in healthy volunteers [41]. It is believed that this effect occurs due to the preferential action of the drug on the NMDA receptors of the GABAergic interneurons compared to those present in pyramidal neurons. Thus, the rapid effect of ketamine is characterized by the deactivation of GABAergic interneurons, preventing its inhibitory effect - via the GABAB receptor [42-44].
The greater affinity of ketamine for NMDAr receptors, expressed on GABAergic interneurons, is mainly due to two factors. One of them would be the high frequency of firing of these cells, which leads to an intracellular polarity conducive to the expulsion of Mg2+, releasing the active site of ketamine in NMDAr. The other would be due to the accentuated expression of the GluN2D subunits in the receptors of these neurons [42-44].
A study in rodents applying MK-801 - another NMDAr antagonist - showed convergent results to those found using ketamine. This assay verified that the drug, firstly, acts on the NMDA receptors of the GABAergic interneurons of the PV class - known as fast-spiking - inhibiting the release of GABA, for, in a second moment, binding to the glutamatergic receptors of pyramidal neurons [45].
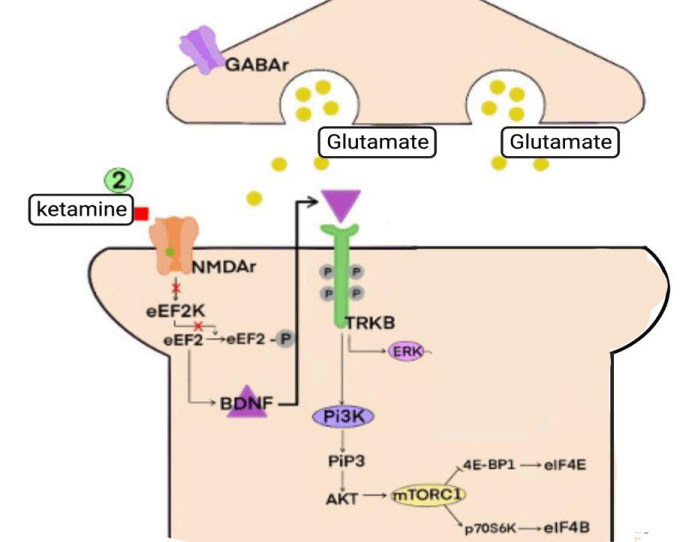
2) NMDA acting directly on Neuroplasticity
Drugs that act as NMDAr antagonists can exert antidepressant effects by modulating different types of synaptic plasticity, including learning processes and long-term potentials (LTP) [46]. For such processes to occur, the presence of some neurotrophins, such as BDNF (brain-derived neurotrophic factor), in addition to the presence of protein kinases, such as mTOR (mammalian target of rapamycin) and eEF2K (eukaryotic elongation factor 2 kinase) - which participate in the translation/transcription of dendritic proteins responsible for cell growth and maintenance of the cytoskeleton - turns into necessary (Figure 5.2) [1,3,12,30,46-50].
The antidepressant effects of ketamine are involved in the modulation of synaptic plasticity by performing an antagonistic action on the NMDAr of spontaneously transmitting chemical synapses. This type of neurotransmission is characterized by the release of neurotransmitters by the presynaptic terminal without a previous action potential, acting differently from the usually evoked neurotransmission [46].
Glutamate release by automatic transmission is related to the activation of the eEF2K/eEF2 signaling pathway by postsynaptic NMDAr. When activated, this pathway inhibits the translation of the BDNF factor - whose presence is necessary for the formation of new synapses and the strengthening of damaged ones [3,46,47].
Two in vivo studies verified that, from the application of sub-venous doses of ketamine, there was an inhibition of the eEF2K/eEF2 pathway, consequently increasing the amount of BDNF present in the analyzed neuron [48,49].
The downstream mechanism carried out by ketamine begins with the deactivation of NMDAr, leading to a decrease in the entry of Ca+2 into the intracellular environment of the postsynaptic neuron. Thus, activating the Ca+2/calmodulin-dependent enzyme eEF2K is impossible, which regulates eEF2 activity by phosphorylating it at Thr56 [48,49]. When dephosphorylated, eEF2 participates in protein translation during the elongation phase, catalyzing the translocation of the tRNA linked to methionine from the A-site to the P-site of the ribosome coupled to the mRNA, thus allowing the addition of a new amino acid in the developing polypeptide chain. One of the polypeptides where the action of eEF2 is necessary for its production is the neurotrophin BDNF [3,46-50].
Among the main neurotrophins existing in the brain of mammals, BDNF is involved with cell survival and differentiation, being, therefore, of great importance for the neuroplastic mechanisms that are impaired in MDD. Studies carried out in animal models that were induced by chronic stress and in individuals with depression verified a decrease in the expression/functioning of BDNF in the regions of the hippocampus and prefrontal cortex, as well as a reduction in the levels of this neurotrophin in the blood plasma in individuals with MDD [1,12,13,51-53].
Ketamine promotes an increase in BDNF production, allowing it to bind to tyrosine kinase B receptors (TRK-B) located in the plasmatic membrane in the form of two inactive monomers, promoting the auto-phosphorylation of these receptors in their tyrosine kinase domains. When activated, TRK-B signals different pathways, such as mitogen-activated protein kinases (MAPK/ERK) and phosphatidylinositol3-kinase/protein kinase B (PI3K/AKT). These, therefore, may activate the mTOR complex [1,54,55].
The mammalian target protein of rapamycin (mTOR) is a serine/threonine kinase, which modulates cell growth, proliferation, and survival; may also act on protein synthesis. Its structure is formed by two polypeptide complexes: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). While mTORC1 stimulates cell proliferation and growth, mTORC2 controls the structure of the cytoskeleton and cell survival [1,54-56].
When activated, the mTORC1 complex can restore protein translation by up-regulating the activity of eukaryotic translation initiation factors. This is made with the inactivating of the factor 4E-BP1 (eukaryotic initiation factor-binding protein 1), the mTORC1 pathway promotes the disinhibition of eIF4E (eukaryotic initiation factor 4E), which can then act again on protein translation [1,54-56].
The mTORC1 complex also promotes polypeptide synthesis by phosphorylating p70S6K protein (70-kDa ribosomal protein S6 kinase), which can then activate eIF4B (eukaryotic initiation factor 4B). When activated, this factor can act on the initiation phase of protein translation [57-60].
After applying a single sub-anesthetic dose of ketamine in rodents, there was an increase in mTOR and p70S6K activity in these animals' prefrontal cortex and hippocampus. Therefore, suggesting that the neuroplastic effects of ketamine are involved with the mTOR signaling pathway [1,57].
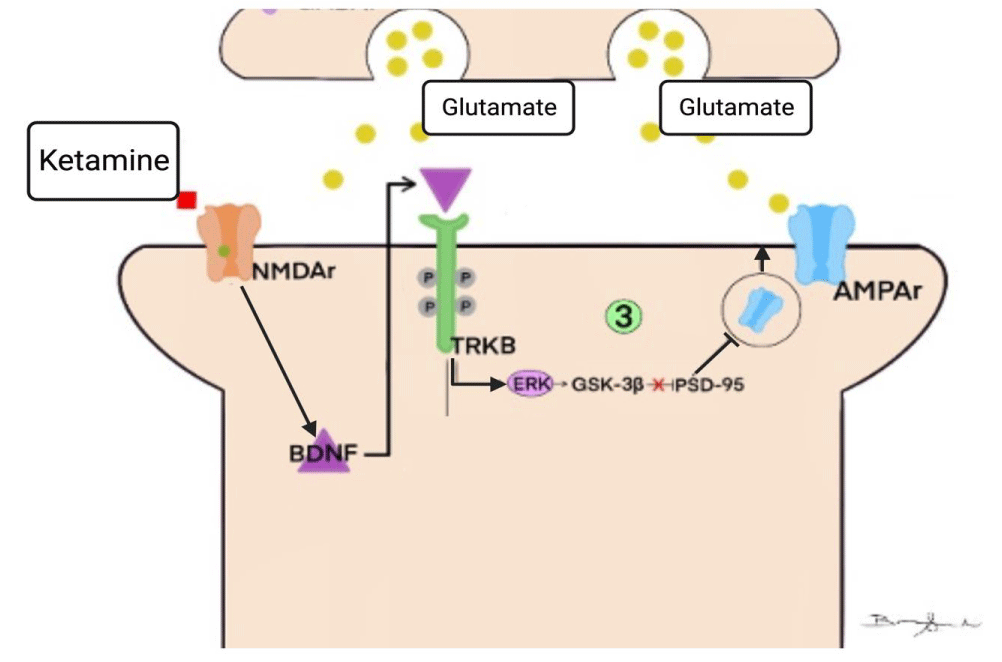
3) The activity of AMPA receptors
AMPA-type glutamatergic receptors (alpha-amino-3-hydroxy-methyl-5-4-isoxazole-propionic) are ligand-dependent ionotropic channels permeable to Na+ and K+ ions and, to a lesser extent, to Ca2+. It is expressed in subunits of four different types (GluA-1, GluA-2, GluA-3, and GluA-4), which organizes in the form of heterotetramers around a central pore. In the postsynaptic membrane of glutamatergic pathways, AMPAr is mainly responsible for the transduction of rapid excitatory signals, as, unlike NMDAr, they do not require a voltage threshold for activation. (Figure 4) [1,32].
AMPA receptors also mediate the phenomenon of Neuroplasticity through the induction and maintenance of LTP and LTD. When LTP is acting, another AMPAr allows into the membrane, and the receptors are phosphorylated, which increases the sensitivity to glutamate in the synapses. On the other hand, when LTD is induced, the reverse process occurs, leading to a decrease in the density and sensitivity of membrane AMPA receptors and a consequent decrease in EPSP (postsynaptic excitatory potential) [1,12,52].
Individuals affected by MDD are deficient in the synaptic communication of glutamatergic excitatory pathways mediated by AMPAr in the hippocampus and PFC regions. The low excitability of these neurons is related to the decrease in the release of the neurotransmitter glutamate by the presynaptic terminal, as well as to an imbalance in the neuroplastic mechanisms - resulting in the favoring of the occurrence of LTD concerning LTP. Through an electrophysiological analysis of the hippocampus, studies carried out in rodents verified that acute stress induces an imbalance of synaptic plasticity, allowing long-term depression to occur mainly in these synapses. This imbalance led to hypofunction, destabilization and neuronal loss [12].
After applications of sub-doses of ketamine, such adverse effects of MDD on synaptic functionality were reversed, and through presynaptic disinhibition, there was greater activation of AMPAr by glutamate. When pre-treatment with the drug NBQX - an AMPAr antagonist - the antidepressant effects of ketamine were almost completely abolished in the analyzed rodents' forced swimming and tail suspension tests. With this, it has increasingly become a consensus among researchers that the rapid antidepressant effect exerted by ketamine is involved with greater activation of AMPAr [1,12,52,53] (Figure 5.3).
The low excitability of AMPAr-mediated glutamatergic synapses in individuals with MDD is also due to the imbalance of neuronal plasticity, which reduces the density of these receptors on dendritic spines. Post-mortem studies have revealed a reduction in the translation of mRNAs that express the AMPAr GluA-1 and GluA-3 subunits in the CA1 region of the hippocampus of individuals with depression, which leads to a lower density of receptors on dendritic spines [12,49] Figure 6.
The antidepressant effect of ketamine is associated with the neuroplastic action of inserting new AMPAr in the postsynaptic membrane through the TrkB/ERK pathway. When the neurotrophin BDNF acts by activating the TrkB receptors, an increase in phosphorylated GSK-3 levels occurs via the ERK pathway. This results in a disinhibition of PSD-95 and, consequently, a greater anchorage of GluA-1 and GluA-2 subunits of AMPAr in the postsynaptic membrane. (Figure 4 and Figure 5.3) Through surface biotinylation studies, that signaling cascade is verified, demonstrating that the synaptic potentiation provided by ketamine accompanies an increase in the expression of GluA-1 and GluA-2 in the membrane. Another experiment shows us that using GluA2-knockout rats in the presence of ketamine allowed us to observe the antidepressant effects of the drug through the non-expression of this AMPAr subunit. Given these findings, the correlation between the antidepressant effects exerted by ketamine and the activity of AMPA receptors becomes increasingly clear [12,49,52].
Conclusion
The elucidation of the neuroplastic approach to depressive disorder opened the way for further research focused on non-aminergic drug therapies to appear. In this sense, this work aimed to highlight the effects of the application of anesthetic sub-doses of ketamine, from a neuro-molecular perspective, in the treatment of MDD.
In conclusion, we can evaluate the main signaling pathways involved in the drug's action in the central nervous system. As a result, the pharmacodynamics of ketamine involves different molecular cascades present in impaired neural plasticity pathways in individuals with MDD. Thus, further research is still needed on the effectiveness of ketamine in treating MDD to consolidate its use in individuals affected by this condition.
- Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018; 23(4):801–11.
- Vecchia DD, Kanazawa LKS, Wendler E, Hocayen PAS, Vital MABF, Takahashi RN, Da Cunha C, Miyoshi E, Andreatini R. Ketamine reversed short-term memory impairment and depressive-like behavior in animal model of Parkinson's disease. Brain Res Bull. 2021 Mar;168:63-73. doi: 10.1016/j.brainresbull.2020.12.011. Epub 2020 Dec 31. PMID: 33359641.
- Carr RM, Oranu A, Khungar V. Antidepressant-like cognitive and behavioral effects of acute ketamine administration associated with plasticity in the ventral hippocampus to medial prefrontal cortex pathway. Physiol Behav. 2016; 176(1):139–48.
- Price RB, Duman R. Neuroplasticity in cognitive and psychological mechanisms of depression: An integrative model. Molecular psychiatry. 2019; 4:1-4.
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, Tye KM, Anikeeva P, Malenka RC, Deisseroth K. Natural neural projection dynamics underlying social behavior. Cell. 2014 Jun 19;157(7):1535-51. doi: 10.1016/j.cell.2014.05.017. PMID: 24949967; PMCID: PMC4123133.
- Grace AA, Bunney BS. Nigral dopamine neurons: intracellular recording and identification with L-dopa injection and histofluorescence. Science. 1980 Nov 7;210(4470):654-6. doi: 10.1126/science.7433992. PMID: 7433992.
- Polter AM, Kauer JA. Stress and VTA synapses: implications for addiction and depression. Eur J Neurosci. 2014 Apr;39(7):1179-88. doi: 10.1111/ejn.12490. PMID: 24712997; PMCID: PMC4019343.
- Morales M, Root DH. Glutamate neurons within the midbrain dopamine regions. Neuroscience. 2014 Dec 12;282:60-8. doi: 10.1016/j.neuroscience.2014.05.032. Epub 2014 May 27. PMID: 24875175; PMCID: PMC4397110.
- Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb Cortex. 2009 Oct;19(10):2479-84. doi: 10.1093/cercor/bhp003. Epub 2009 Feb 4. PMID: 19193712; PMCID: PMC2742599.
- Zhang M, Radford KD, Driscoll M, Purnomo S, Kim J, Choi KH. Effects of subanesthetic intravenous ketamine infusion on Neuroplasticity-related proteins in the prefrontal cortex, amygdala, and hippocampus of Sprague-Dawley rats. IBRO Rep. 2019 Jan 16;6:87-94. doi: 10.1016/j.ibror.2019.01.006. PMID: 30723838; PMCID: PMC6350099.
- Becker B, Steffens M, Zhao Z, Kendrick KM, Neumann C, Weber B, Schultz J, Mehta MA, Ettinger U, Hurlemann R. General and emotion-specific neural effects of ketamine during emotional memory formation. Neuroimage. 2017 Apr 15;150:308-317. doi: 10.1016/j.neuroimage.2017.02.049. Epub 2017 Feb 20. PMID: 28232170.
- Aleksandrova LR, Phillips AG, Wang YT. Antidepressant effects of ketamine and the roles of AMPA glutamate receptors and other mechanisms beyond NMDA receptor antagonism. J Psychiatry Neurosci. 2017 Jun;42(4):222-229. doi: 10.1503/jpn.160175. PMID: 28234212; PMCID: PMC5487269.
- Wu C, Wang Y, He Y, Wu S, Xie Z, Zhang J, Shen J, Wang Z, He L. Sub-anesthetic and anesthetic ketamine produce different long-lasting behavioral phenotypes (24 h post-treatment) via inducing different brain-derived neurotrophic factor (BDNF) expression level in the hippocampus. Neurobiol Learn Mem. 2020 Jan;167:107136. doi: 10.1016/j.nlm.2019.107136. Epub 2019 Dec 5. PMID: 31812581.
- Lerner SE, Burns RS. Phencyclidine use among youth: history, epidemiology, and acute and chronic intoxication. NIDA Res Monogr. 1978 Aug;(21):66-118. PMID: 101877.
- Bertron JL, Seto M, Lindsley CW. DARK Classics in Chemical Neuroscience: Phencyclidine (PCP). ACS Chem Neurosci. 2018 Oct 17;9(10):2459-2474. doi: 10.1021/acschemneuro.8b00266. Epub 2018 Jul 17. PMID: 29953199.
- Williams JF, Lundahl LH. Focus on Adolescent Use of Club Drugs and "Other" Substances. Pediatr Clin North Am. 2019 Dec;66(6):1121-1134. doi: 10.1016/j.pcl.2019.08.013. PMID: 31679602.
- Morgan CJ, Curran HV; Independent Scientific Committee on Drugs. Ketamine use: a review. Addiction. 2012 Jan;107(1):27-38. doi: 10.1111/j.1360-0443.2011.03576.x. Epub 2011 Jul 22. PMID: 21777321.
- Kalsi SS, Wood DM, Dargan PI. The epidemiology and patterns of acute and chronic toxicity associated with recreational ketamine use. Emerg Health Threats J. 2011 Apr 15;4:7107. doi: 10.3402/ehtj.v4i0.7107. PMID: 24149025; PMCID: PMC3168228.
- Jessica N. Facing addiction in America: The surgeon general’s report on alcohol, drugs, and health: A commentary. Alcoholism Treatment Quarterly. 35.4 (2017): 445-454.
- United Nations Office on Drugs and Crime. World drug report 2019. 2019. https://www.drugsandalcohol.ie/30717/.
- Vines L, Sotelo D, Johnson A, Dennis E, Manza P, Volkow ND, Wang GJ. Ketamine use disorder: preclinical, clinical, and neuroimaging evidence to support proposed mechanisms of actions. Intell Med. 2022 May;2(2):61-68. doi: 10.1016/j.imed.2022.03.001. Epub 2022 Mar 7. PMID: 35783539; PMCID: PMC9249268.
- Le TT, Cordero IP, Jawad MY, Swainson J, Di Vincenzo JD, Jaberi S, Phan L, Lui LMW, Ho R, Rosenblat JD, McIntyre RS. The abuse liability of ketamine: A scoping review of preclinical and clinical studies. J Psychiatr Res. 2022 Jul;151:476-496. doi: 10.1016/j.jpsychires.2022.04.035. Epub 2022 May 10. PMID: 35623124.
- Bonaventura J, Lam S, Carlton M, Boehm MA, Gomez JL, Solís O, Sánchez-Soto M, Morris PJ, Fredriksson I, Thomas CJ, Sibley DR, Shaham Y, Zarate CA Jr, Michaelides M. Pharmacological and behavioral divergence of ketamine enantiomers: implications for abuse liability. Mol Psychiatry. 2021 Nov;26(11):6704-6722. doi: 10.1038/s41380-021-01093-2. Epub 2021 Apr 15. PMID: 33859356; PMCID: PMC8517038.
- Leal GC, Bandeira ID, Correia-Melo FS, Telles M, Mello RP, Vieira F, Lima CS, Jesus-Nunes AP, Guerreiro-Costa LNF, Marback RF, Caliman-Fontes AT, Marques BLS, Bezerra MLO, Dias-Neto AL, Silva SS, Sampaio AS, Sanacora G, Turecki G, Loo C, Lacerda ALT, Quarantini LC. Intravenous arketamine for treatment-resistant depression: open-label pilot study. Eur Arch Psychiatry Clin Neurosci. 2021 Apr;271(3):577-582. doi: 10.1007/s00406-020-01110-5. Epub 2020 Feb 20. PMID: 32078034.
- Jones JL, Mateus CF, Malcolm RJ, Brady KT, Back SE. Efficacy of Ketamine in the Treatment of Substance Use Disorders: A Systematic Review. Front Psychiatry. 2018 Jul 24;9:277. doi: 10.3389/fpsyt.2018.00277. PMID: 30140240; PMCID: PMC6094990.
- Phillips JL, Norris S, Talbot J, Birmingham M, Hatchard T, Ortiz A, Owoeye O, Batten LA, Blier P. Single, Repeated, and Maintenance Ketamine Infusions for Treatment-Resistant Depression: A Randomized Controlled Trial. Am J Psychiatry. 2019 May 1;176(5):401-409. doi: 10.1176/appi.ajp.2018.18070834. Epub 2019 Mar 29. PMID: 30922101.
- Food, U. S. "Drug Administration. FDA approves new nasal spray medication for treatment-resistant depression; available only at a certified doctor’s office or clinic." FDA News Release. 2019; 3.
- McIntyre RS, Rosenblat JD, Nemeroff CB, Sanacora G, Murrough JW, Berk M, Brietzke E, Dodd S, Gorwood P, Ho R, Iosifescu DV, Lopez Jaramillo C, Kasper S, Kratiuk K, Lee JG, Lee Y, Lui LMW, Mansur RB, Papakostas GI, Subramaniapillai M, Thase M, Vieta E, Young AH, Zarate CA Jr, Stahl S. Synthesizing the Evidence for Ketamine and Esketamine in Treatment-Resistant Depression: An International Expert Opinion on the Available Evidence and Implementation. Am J Psychiatry. 2021 May 1;178(5):383-399. doi: 10.1176/appi.ajp.2020.20081251. Epub 2021 Mar 17. PMID: 33726522; PMCID: PMC9635017.
- Initiates Phase, Perception Neuroscience. "2a study of PCN-101 (R-ketamine) for treatment resistant depression.[Press release] https://ir. atai. Life/news-releases/news-release-details/perception-neuroscience-initiates-phase-2a-study-pcn-101-r. 2021.
- Prybylowski K, Wenthold RJ. N-Methyl-D-aspartate receptors: subunit assembly and trafficking to the synapse. J Biol Chem. 2004 Mar 12;279(11):9673-6. doi: 10.1074/jbc.R300029200. Epub 2004 Jan 23. PMID: 14742424.
- Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav Brain Res. 2003 Mar 18;140(1-2):1-47. doi: 10.1016/s0166-4328(02)00272-3. PMID: 12644276.
- Kandel ER, Schwartz JH. Princípios da Neurociência, Manole, 4ed., 2003;
- Kreutz Michael R, Sala Carlo. Synaptic Plasticity: Dynamics, Development and Disease. 1st ed. 2012;
- Phelps CH. Neural plasticity in aging and Alzheimer's disease: some selected comments. Prog Brain Res. 1990;86:3-9. doi: 10.1016/s0079-6123(08)63162-3. PMID: 2087561.
- Kolb B, Whishaw IQ. Plasticity in the neocortex: mechanisms underlying recovery from early brain damage. Prog Neurobiol. 1989;32(4):235-76. doi: 10.1016/0301-0082(89)90023-3. PMID: 2655008.
- Skolnick P, Layer RT, Popik P, Nowak G, Paul IA, Trullas R. Adaptation of N-methyl-D-aspartate (NMDA) receptors following antidepressant treatment: implications for the pharmacotherapy of depression. Pharmacopsychiatry. 1996 Jan;29(1):23-6. doi: 10.1055/s-2007-979537. PMID: 8852530.
- Vithlani M, Terunuma M, Moss SJ. The dynamic modulation of GABA(A) receptor trafficking and its role in regulating the plasticity of inhibitory synapses. Physiol Rev. 2011 Jul;91(3):1009-22. doi: 10.1152/physrev.00015.2010. PMID: 21742794; PMCID: PMC4382539.
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004 Jul;84(3):835-67. doi: 10.1152/physrev.00036.2003. PMID: 15269338.
- Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M, Bonner TI, Enna SJ. International Union of Pharmacology. XXXIII. Mammalian gamma-aminobutyric acid(B) receptors: structure and function. Pharmacol Rev. 2002 Jun;54(2):247-64. doi: 10.1124/pr.54.2.247. PMID: 12037141.
- Cryan JF, Kaupmann K. Don't worry 'B' happy!: a role for GABA(B) receptors in anxiety and depression. Trends Pharmacol Sci. 2005 Jan;26(1):36-43. doi: 10.1016/j.tips.2004.11.004. PMID: 15629203.
- Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D. Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiatry. 1997 Jun;154(6):805-11. doi: 10.1176/ajp.154.6.805. PMID: 9167508.
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997 Apr 15;17(8):2921-7. doi: 10.1523/JNEUROSCI.17-08-02921.1997. PMID: 9092613; PMCID: PMC6573099.
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994 Mar;12(3):529-40. doi: 10.1016/0896-6273(94)90210-0. PMID: 7512349.
- Perszyk RE, DiRaddo JO, Strong KL, Low CM, Ogden KK, Khatri A, Vargish GA, Pelkey KA, Tricoire L, Liotta DC, Smith Y, McBain CJ, Traynelis SF. GluN2D-Containing N-methyl-d-Aspartate Receptors Mediate Synaptic Transmission in Hippocampal Interneurons and Regulate Interneuron Activity. Mol Pharmacol. 2016 Dec;90(6):689-702. doi: 10.1124/mol.116.105130. Epub 2016 Sep 13. PMID: 27625038; PMCID: PMC5118640.
- Neske GT, Patrick SL, Connors BW. Contributions of diverse excitatory and inhibitory neurons to recurrent network activity in cerebral cortex. J Neurosci. 2015 Jan 21;35(3):1089-105. doi: 10.1523/JNEUROSCI.2279-14.2015. PMID: 25609625; PMCID: PMC4300319.
- Monteggia LM, Gideons E, Kavalali ET. The role of eukaryotic elongation factor 2 kinase in rapid antidepressant action of ketamine. Biol Psychiatry. 2013 Jun 15;73(12):1199-203. doi: 10.1016/j.biopsych.2012.09.006. Epub 2012 Oct 11. PMID: 23062356; PMCID: PMC3574622.
- Heise C, Gardoni F, Culotta L, di Luca M, Verpelli C, Sala C. Elongation factor-2 phosphorylation in dendrites and the regulation of dendritic mRNA translation in neurons. Front Cell Neurosci. 2014 Feb 10;8:35. doi: 10.3389/fncel.2014.00035. PMID: 24574971; PMCID: PMC3918593.
- Grønli J, Dagestad G, Milde AM, Murison R, Bramham CR. Post-transcriptional effects and interactions between chronic mild stress and acute sleep deprivation: regulation of translation factor and cytoplasmic polyadenylation element-binding protein phosphorylation. Behav Brain Res. 2012 Dec 1;235(2):251-62. doi: 10.1016/j.bbr.2012.08.008. Epub 2012 Aug 16. PMID: 22917528.
- Nosyreva E, Szabla K, Autry AE, Ryazanov AG, Monteggia LM, Kavalali ET. Acute suppression of spontaneous neurotransmission drives synaptic potentiation. J Neurosci. 2013 Apr 17;33(16):6990-7002. doi: 10.1523/JNEUROSCI.4998-12.2013. PMID: 23595756; PMCID: PMC3661220.
- Abelaira Helena Mendes. O papel da via mTOR no efeito antidepressivo da cetamina [Tese]. Santa Catarina: Universidade do Extremo Sul Catarinense - UNESC; 2017.
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011 Jun 15;475(7354):91-5. doi: 10.1038/nature10130. PMID: 21677641; PMCID: PMC3172695.
- Peng FZ, Fan J, Ge TT, Liu QQ, Li BJ. Rapid anti-depressant-like effects of ketamine and other candidates: Molecular and cellular mechanisms. Cell Prolif. 2020 May;53(5):e12804. doi: 10.1111/cpr.12804. Epub 2020 Apr 7. PMID: 32266752; PMCID: PMC7260066.
- Cavalleri L, Merlo Pich E, Millan MJ, Chiamulera C, Kunath T, Spano PF, Collo G. Ketamine enhances structural plasticity in mouse mesencephalic and human iPSC-derived dopaminergic neurons via AMPAR-driven BDNF and mTOR signaling. Mol Psychiatry. 2018 Apr;23(4):812-823. doi: 10.1038/mp.2017.241. Epub 2017 Nov 21. PMID: 29158584.
- Abelaira HM, Réus GZ, Neotti MV, Quevedo J. The role of mTOR in depression and antidepressant responses. Life Sci. 2014 Apr 17;101(1-2):10-4. doi: 10.1016/j.lfs.2014.02.014. Epub 2014 Feb 25. PMID: 24582593.
- Abelaira HM, Réus GZ, Ignácio ZM, Dos Santos MA, de Moura AB, Matos D, Demo JP, da Silva JB, Michels M, Abatti M, Sonai B, Dal Pizzol F, Carvalho AF, Quevedo J. Effects of ketamine administration on mTOR and reticulum stress signaling pathways in the brain after the infusion of rapamycin into prefrontal cortex. J Psychiatr Res. 2017 Apr;87:81-87. doi: 10.1016/j.jpsychires.2016.12.002. Epub 2016 Dec 3. PMID: 28017918.
- Qiao J, Sun Y, Wu J, Wang L. Investigation of the underling mechanism of ketamine for antidepressant effects in treatment-refractory affective disorders via molecular profile analysis. Exp Ther Med. 2019 Jul;18(1):580-588. doi: 10.3892/etm.2019.7633. Epub 2019 May 30. PMID: 31281445; PMCID: PMC6580107.
- Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, Karolewicz B. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011 Aug 15;35(7):1774-9. doi: 10.1016/j.pnpbp.2011.05.010. Epub 2011 May 23. PMID: 21635931; PMCID: PMC3154612.
- Sobolevsky AI, Rosconi MP, Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009 Dec 10;462(7274):745-56. doi: 10.1038/nature08624. PMID: 19946266; PMCID: PMC2861655.
- Miller PS, Aricescu AR. Crystal structure of a human GABAA receptor. Nature. 2014 Aug 21;512(7514):270-5. doi: 10.1038/nature13293. Epub 2014 Jun 8. PMID: 24909990; PMCID: PMC4167603.
- Zhang Y, Ye F, Zhang T, Lv S, Zhou L, Du D, Lin H, Guo F, Luo C, Zhu S. Structural basis of ketamine action on human NMDA receptors. Nature. 2021 Aug;596(7871):301-305. doi: 10.1038/s41586-021-03769-9. Epub 2021 Jul 28. Erratum in: Nature. 2021 Oct;598(7882):E3. PMID: 34321660.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
