Archives of Depression and Anxiety
The effect of low-pressure blast-wave exposure on middle aged rats
Galya Herzog1, Amitai Zuckerman2,3, Omri Ram4, Michael A Matar2,3, Zeev Kaplan2,3, Amir B Geva5, Oren Sadot4 and Hagit Cohen1-3*
2Faculty of Health Sciences, Ben-Gurion University of the Negev, Israel
3Ministry of Health, Anxiety and Stress Research Unit, Beer-Sheva Mental Health Center, Israel
4Department of Mechanical Engineering Ben-Gurion University, Israel
5Department of Electrical and Computer Engineering, Ben Gurion University of the Negev, Beer-Sheva, Israel
Cite this as
Herzog G, Zuckerman A, Ram O, Matar MA, Cohen H, et al. (2020) The effect of low-pressure blast-wave exposure on middle aged rats. Arch Depress Anxiety 6(2): 066-078. DOI: 10.17352/2455-5460.000055The objective of this study was to explore how age at the time of blast-exposure affects behavioral and cognitive responses. Non-anesthetized male middle-aged rats were exposed to visual, auditory, olfactory, and tactile effects of a low-pressure explosive blast-wave produced by exploding a thin copper wire. Validated cognitive and behavioral paradigms were used to assess both the PTSD-phenotype and mTBI-phenotype. Naïve middle-aged rats displayed very heterogeneous individual responses. Whereas some middle-aged rats performed as well as young rats, others showed pronounced cognitive deficits and several were unable to find the platform at all. The variance made it difficult to determine "normal" criteria for learning patterns. The middle-aged rats displayed significantly worse behavioral outcomes following blast-exposure than young rats. This finding was especially evident in depression-related behavior, and there was a significant decline in spatial reference learning ability, which was not observed in younger rats. These results indicate that middle-aged rats respond differently to blast exposure than young rats and that age is an important factor to consider in pre-clinical efficacy studies. This study emphasizes the complexity of working with older subjects, both in terms of determining "baseline norms" and in terms of the pattern of responses to the experimental paradigm. These characteristics are also found in studies involving older human subjects, certainly in terms of age-related baseline characteristics.
Introduction
Exposure to explosive detonations of conventional or improvised explosives in battle and terror attacks is quite common [1,2]. Explosive detonations generate an expanding blast-wave characterized by a rapid initial impulse of atmospheric overpressure followed by an exponential decay leading to under-pressure, which causes a reverse blast wind toward the under-pressured area [3]. Explosions in populated areas expose individuals to the risk of injury and death and to the traumatizing experiences of witnessing the outcome of the explosion.
Recently, we developed a research paradigm for a single low-intensity blast-wave exposure that causes minimal traumatic brain injury (mTBI), and models the traumatic experiences of exposure to improvised explosive devices and/or other battlefield-related blast-waves [4,5]. The apparatus developed for this purpose generates a blast-wave through the detonation of a thin copper wire knotted to approximate the spherical blast-wave commonly generated by improvised or standard explosive devices [4]. The detonation involves a bright flash, loud bang, odorous smoke, and tangible low-pressure blast-wave with an appropriate overpressure peak and under-pressure profile, devoid of artifactual flow patterns not encountered in real situations [4]. Similar to what occurs on the battlefield, we found a degree of heterogeneity in individual responses to this blast exposure. Although most animals exposed to the blast did not demonstrate any behavioral or cognitive abnormalities, a significant number of rats exhibited behaviors resembling either a PTSD phenotype, mTBI phenotype, or a combined PTSD-mTBI phenotype [4,5]. While PTSD and mTBI are both well-described in the general population, the literature concerning PTSD and/or mTBI in the elderly is sparse and patchy. There are indications that both PTSD and mTBI in old age present with unique features. There are indications that increased age at the time of TBI exposure is associated with increased likelihood of negative neurological outcomes and immune response in rats [6] or with increased mortality and hospitalization rates, as well as worse functional outcomes, in humans [7,8].
The overall goal of this study was to explore how age at the time of blast exposure injury affects behavioral and cognitive responses in multiple neurological and behavioral domains over an extended time course. In the present study, we initially exposed middle-aged rats to a controlled low-pressure blast-wave. We assessed behavioral and cognitive responses in exposed rats in order to identify possible distinguishing behaviors related to mTBI, in comparison to well-established stress-induced (PTSD-like) behavioral responses. We assessed possible criteria for data analysis for mTBI-related behaviors to complement the well-validated cutoff behavioral criteria that serve in the analysis of data reflecting PTSD-like responses in our studies [9].
Since age at the time of blast exposure is likely to influence the way the brain is able to respond as a result of developmental status, extent of cellular senescence, and injury-induced inflammation [10], it was hypothesized that middle-aged rats will have worse outcomes than their younger counterparts.
Methods
All procedures were performed under strict compliance with the ethical principles and guidelines of the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. All treatment and testing procedures were approved by the Animal Care Committee of Ben-Gurion University of the Negev, Israel (IL-77-12-2016). All efforts were made to minimize the number of animals used and their suffering.
Animals
A total of 149 male Sprague-Dawley rats (Envigo Laboratories, Israel) aged 12–13 months old, weighing 400–750 g were used in this study. The animals were housed two per cage in a vivarium with a stable temperature and 12-hour light/dark cycle (lights off: 08:00 a.m.) with unlimited access to food and water. All efforts were made to minimize the number of animals used and their suffering.
Experimental design
Rats were randomly assigned into three groups: 1) blast-exposed group (n=65), 2) sham-exposed group (n=20), and 3) control unexposed group (n=64). Rats were tested in batches of 5–10 rats. Rats were exposed to a low-pressure blast-wave (described subsequently) or a sham procedure. Neurological assessment using the neurological severity score (NSS) was performed one hour after the blast and daily thereafter. During the experiment, the rats were weighed every 3 days to assess whether there was any weight loss that could indicate depression-like behavior. Other behavioral measures were conducted on day seven. The rats were initially assessed in the elevated plus maze followed by using the acoustic startle response paradigm 1 h later. Spatial memory performance using the Morris water-maze test was assessed at 8 days post-exposure. One day after the last day of the Morris water maze, the rats underwent the forced swimming test to assess depression. Rats were sacrificed 24 h after the completion of the Morris water-maze test to undergo morphology tests. The prevalence rates of PTSD-like and mTBI-like responses were calculated from these data and compared with those of sham-exposed controls and unexposed rats. The experimental design is shown in Figure 1.
Blast-wave exposure
The experiment used an exploding wire technique to generate small-scale cylindrical and spherical blast-waves. This technique has been previously demonstrated to simulate the effects of air blast exposure under experimental conditions and permits safe operation with high repeatability [4,5,11].
Exploding wires
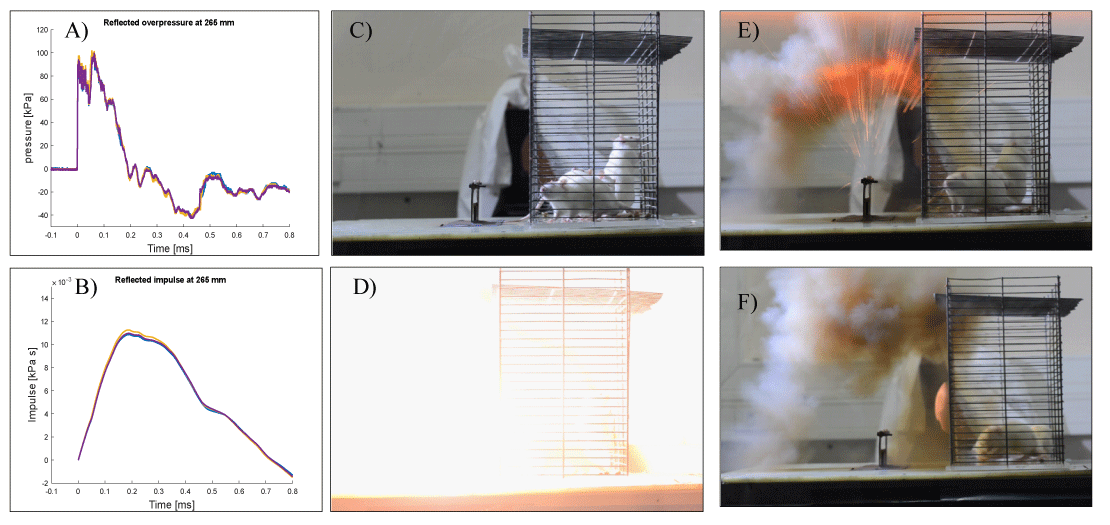
To initiate a low blast explosion, a current was created by a high-voltage power supply generated from a capacitor, which was delivered to a thin wire. When the short, high-current pulse passes through the thin conducting wire, the latter undergoes rapid heating and expansion, and then evaporates. The rapid expansion generates a strong blast-wave, whose strength is controlled by the charging voltage. This method of explosion produces a cylindrical blast-wave that simulates a blast-wave profile similar to that seen from explosive devices frequently seen on the battlefield. In an actual explosion, the blast-wave causes an acute, short-duration elevation in pressure followed by a negative phase. The exploding wire system has been shown to be capable of duplicating this overpressure and under-pressure blast-wave profile (Figure 2A-B). This technique has been previously demonstrated to simulate the effects of air blast exposure under experimental conditions and permits safe operation with high repeatability [11-13].
Procedure
Every explosion involved two rats that were separated in one big cage (Figure 2C-F). The cage was then placed in the blast-wave generation system 265 mm from the wire. Pressure values were recorded using a Kistler 211B3 piezoelectric pressure transducer mounted on a perpendicular wall. Rats were subjected to a single blast-wave with their head facing the blast without shielding following; the rats were then returned to their home cage.
Sham exposure (control)
Sham-exposed animals were treated identically except that they did not receive a blast exposure. The rats were in the same room where the explosion took place but were not exposed to the blast-wave (they were shielded sitting ∼150 cm below the blast-wave). This enabled us to focus on emotional components without the compounding effects of physical brain injuries.
Neurological Severity Score (NSS)
In order to ensure that any damage to the Central Nervous System (CNS) caused by the blast-wave did not result in vast neurological deficits, we employed the NSS. The NSS was performed 1-h following the initial blast wave exposure and served as a baseline for comparison with later evaluations throughout the study [14]. NSS assesses somatomotor and somatosensory function by evaluating the animals’ activities in motor, sensory, reflexes, beam walking, and beam balancing tasks. Specifically, the following were assessed: ability to exit from a circle (3- point scale), gait on a wide surface (3-point scale), gait on a narrow surface (4-point scale), effort to remain on a narrow surface (2-point scale), reflexes (5-point scale), seeking behavior (2-point scale), beam walking (3-point scale), and beam balance (3- point scale). An observer, who was blind to the different treatment groups, tested the animals.
Behavioral paradigms
All rats underwent a number of different behavioral assessments. All behavioral tests were performed in a closed, quiet, light-controlled room in the Faculty of Medicine, Anxiety and Stress Research Unit, Ben-Gurion University between 10:00 and 16:00. No animals underwent the same test twice. All behavioral tests were video-recorded for future analysis using the ETHO-VISION program (Noldus). The behavioral tests included the Elevated Plus Maze (EPM) and Acoustic Startle Response (ASR) for anxiety-like/PTSD-like responses, forced swimming test for depression, and Morris Water Maze (MWM) for cognitive performance.
Elevated plus maze
The maze is a plus-shaped platform with two opposing open arms, and two opposing closed arms (surrounded by 14cm high opaque walls on three sides) (File, 1993). Rats were placed on the central platform facing an open arm and allowed to explore the maze for 5 min. Each test was videotaped and subsequently scored by an independent observer. Arm entry was defined as entering an arm with all four paws. Behaviors assessed were: time spent (duration) in open and closed arms and on the central platform, number of open arms entries, and total exploration (entries into all arms). Total exploration was calculated as the number of entries into any arm of the maze in order to distinguish between impaired exploratory behavior, exploration limited to closed arms (avoidance), and free exploration. ‘‘Anxiety Index,’’ an index that integrates the elevated plus maze behavioral measures, was calculated as follows:
Anxiety Index values range from 0 to 1, where an increase in the index expresses increased anxiety-like behavior (Cohen, et al. 2008a; Cohen, et al. 2012; Cohen, et al. 2008b).
Startle response was measured using two ventilated startle chambers (SR-LAB system, San Diego Instruments, San Diego, CA). The SR-LAB calibration unit was used routinely to ensure consistent stabilimeter sensitivity between test chambers and over time. Each Plexiglass cylinder rests on a platform inside a sound-proofed, ventilated chamber. Movement inside the tube is detected by a piezoelectric accelerometer below the frame. Sound levels within each test chamber are measured routinely using a sound level meter (Radio Shack) to ensure consistent presentation. Each test session started with a 5min acclimatization period to background white noise of 68dB, followed by 30 acoustic startle trial stimuli in six blocks (110 dB white noise of 40ms duration with 30 or 45sec inter-trial interval). Behavioral assessments consisted of: mean startle amplitude (average over all 30 trials) and percent of startle habituation to repeated presentation of the acoustic pulse. Percent habituation – the percent change was calculated between the response to the first and last (6th) blocks of sound stimuli, as follows:
Forced swimming test
The forced swimming test is a standard behavioral test for assessing depression in rodents, and it is used to test the efficiency of antidepressant drugs (Porsolt, et al. 2001; Porsolt, et al. 1977). The forced swimming test was conducted by placing rats in individual glass cylinders (50 cm tall and 20 cm in diameter) containing room temperature water at a depth of 40 cm. To acclimate the rats to the protocol, they were placed in the glass cylinder for a 15min swimming session. No data collection occurred during this session. Following the swim, rats were removed from the cylinders, dried with paper towels, placed in heated cages for 15min, and then returned to their cages. On the subsequent day, 24h following the acclimation trial, a 5min test was performed, and behavior was digitally recorded for assessment. After the 5min test, the rats were dried and were returned to their cages. Experiments were videotaped for post-recording measurements of the test, duration of immobility periods, and climbing activity. Immobility was defined as when the rat remained afloat in the water without struggling, and only made movements that were necessary to keep its head above water (Detke, Rickels, & Lucki, 1995; Slattery & Cryan, 2012).
MWM
Spatial learning and memory were assessed by performance in a hippocampal-dependent visuospatial learning task in the Morris water maze according to a test modified from the procedure of Morris (Morris, 1984). The rats were trained in a pool 1.8m in diameter and 0.6m high, filled half way with water at 24–1C. A transparent platform was hidden in a constant position in the pool submerged 1cm below the water level. Within the testing room, only distal visuospatial cues were available to the rats for location of the submerged platform. Rats were given four trials per day to find the hidden platform over 4 consecutive days (acquisition phase). The escape latency, (i.e., the time required by the rat to find and climb onto the platform), was recorded for up to 120sec. Each rat was allowed to remain on the platform for 30sec and was then removed to its home cage. If the rat did not find the platform within 120sec, it was manually placed on the platform and was returned to its home cage after 30sec. To assess reference memory at the end of learning, a probe trial was given. Twenty-four hours after the last acquisition day, the submerged platform was removed, and the search strategy of the rat was monitored to evaluate whether it used spatial memory to search for the submerged platform in the quadrant where it had previously been located. On days 6-7, the platform was placed at the opposite end of the pool, and the rat was re-trained in four daily sessions (reversal phase). All groups were tested in the MWM relatively early (Days 8–11 post-exposure, the acquisition trials; Day 12 post-exposure, probe trial; and Days 13–15 post-exposure, the reversal trials).
Retrospective classifications and re-analyses by response pattern
Cutoff behavioral criteria model of PTSD: To model the Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria for PTSD, we developed a cutoff behavioral criteria model of the PTSD-like phenotype [15-19]. The model was originally motivated by the fact that the clinical diagnosis of PTSD is made only if an individual exhibits a certain number of symptoms of sufficient severity from each of the well-defined symptom clusters over a specific period of time. The classification of individuals according to the degree to which their individual behavior is affected by a stressor is based on the premise that in the natural environment, such extremely compromised behavior in response to a priming trigger may compromise behaviors essential for survival, and is, therefore, inadequate and maladaptive, representing a pathological degree of response. The criteria were based on the EPM and ASR paradigms, and clearly define two opposing extremes of individual responses. The behavioral responses of animals in both the EPM and ASR tests were first analyzed according to study group (i.e., stress-exposed vs. controls) in the usual manner. Subsequently, individual animals were classified according to their behavioral response pattern on both the EPM and ASR by using the cutoff behavioral criteria model as exhibiting either ‘‘extreme behavioral response’’ (EBR) or ‘‘minimal behavioral response’’ (MBR). Those that fulfilled neither set of criteria were labeled as exhibiting a ‘‘partial behavioral response’’ (PBR). The detailed protocols are described in Supplementary Information 1.
Cognitive performance criteria model of mTBI
Memory loss or impairment is one criterion for human mTBI; therefore, we used the MWM paradigm to evaluate learning and memory performance in this study [20]. Traditionally, comparisons between crude performance measures have been used to assess learning and memory between groups, such as escape latencies, time spent in different areas of the pool, or swim speed. These analyses are limited by individual variability among subjects. Intrinsic variation within treatment groups arguably functions as a major barrier prohibiting statistically conclusive results, provoking false negatives that may mask true differences. It is well known in human studies that only a proportion of the population exposed to a blast-wave develops symptoms fulfilling mTBI criteria [1, 21, 22]. Individual variations in the response of blast-wave animals are also similar, suggesting that certain segments of a population may be more intrinsically protected from injury from a low-intensity blast wave. Therefore, clearly defined and reliable diagnostic criteria for animal responses would augment the clinical validity of the analysis of the study data. As such, in previous studies we designed (inclusion/exclusion) cognitive criteria that classify study subjects as being affected by the blast-wave exposure to be applied retrospectively in the analysis of behavioral data. The performance was analyzed using the exponential decay model [9]. After analyzing our results, the variance made it difficult to determine "normal" criteria for learning patterns in middle age with this model; therefore, we used dynamic Unsupervised hierarchical Fuzzy Clustering (UFC) to re-assess our results.
Unsupervised fuzzy clustering analysis
UFC is a mathematical technique that groups together objects in multidimensional feature space according to a specified similarity measurement, thereby yielding clusters of similar data points that can be represented by a set of prototypes or centroids [23,24]. We set out to re-process the raw data to examine whether UFC analysis would group the animals in a similar manner to cognitive grouping.
Statistical analyses
All data are reported as mean ± stand error. A level of p<0.05 was used to determine statistical significance. For the behavioral and molecular results, the statistical analyses were performed using two-way analysis of variance (ANOVA) (three-way for Sholl-analysis). Post-hoc Bonferroni tests examined differences between individual groups. A general linear model (repeated-measures ANOVA) was used to analyze the MWM data. The Area Under the Curve (AUC) for time spent in the Morris water maze was calculated using the trapezoidal method. After these calculations, the observed variance was very high. Consequently, we used dynamic UFC. The prevalence of affected rats as a function of rat group was tested using cross-tabulation and nonparametric χ2 tests. In all cases, p<0.05 was considered statistically significant.
Results
There was no mortality in any of the blast-exposed, sham-exposed, or control groups. No significant differences in body weight were found between groups, and weight gain during the 17 days post-blast exposure was comparable in all groups.
Blast-wave details: A 0.8-mm diameter, 70-mm long copper wire, and charging voltage of 4.2 kV were used to generate the blast-wave. The discharge current was 500 kA (estimated). The short pulse at t = 0 is associated with the electromagnetic pulse generated by the capacitor discharge. Animals subjected to the blast-wave experienced a mean peak overpressure of 95 kPa (13.77 psi) (rise time of 0.01 ms) sustained for a duration of 0.189 ms. The peak pressure was equivalent to 193 dB Sound Pressure Level (SPL). The exposure led to a peak impulse of 10.8 × 10-3 kPa-s. (Figure 2A-B). A negative pressure was sustained for >0.659 ms with a peak negative pressure of -40 kPa (-5.8 psi). The light intensity generated by the explosion was significant and measured to be approximately 5 Mlux. This light intensity is of the same order of magnitude as that of the M84 stun grenade at a distance of 1.5 m (3.1 Mlux). The repeatability of the system is demonstrated in Figure 2A-B, where three different experiments are superimposed. It was found that the peak overpressure deviations between the different experiments were between 1% and 3%. To the best of our knowledge, this could not be achieved with TNT explosions.
Neurological severity score: There were no significant differences between the groups in reflex responses, motor coordination, motor strength, or sensory function (data not shown). All animals were graded as zero, suggesting that differences between the behavioral and cognitive tasks were not related to abnormal motor function required to complete the behavioral tasks.
Effects of low-pressure blast exposure on behavior
Elevated plus maze: Exposure to either the blast-wave or the sham conditions significantly increased anxiety-like behavior relative to the unexposed controls (Figure 3). A significant decrease was observed in the time spent in the open arms (F [2,146] =5.1, p<0.00735) in the exposed (blast-wave and sham) groups compared with that in the unexposed group (p<0.035 and p<0.03, respectively). No significant decrease was observed in the time spent in the open arms in the blast-wave-exposed group compared with that in the sham-exposed group (Figure 3A). Following blast-wave exposure, a significant decrease was observed in the number of entries to the open arms (F[2,146]=5.5, p<0.0055) in the blast-wave-exposed groups compared with that in the unexposed controls (p<0.005) (Figure. 3B). A significant increase was observed in the time spent in the central platform (F[2,146]=14.5, p<0.0001) in the exposed (blast-wave and sham) groups compared with that in the unexposed group (p<0.02 and p<0.0001, respectively). In addition, a significant increase was observed in the time spent in the central platform in the sham-exposed group compared with that in the blast-wave-exposed group (p<0.003) (Figure 3C). Complementing these findings, the anxiety index was significantly increased in both the blast-wave and sham-exposure groups compared with that in the unexposed controls (F[2,95]=6.7, p<0.002). No significant differences were observed in the anxiety index between the blast-wave and sham-exposed groups (Figure 3E). There were no differences in total exploration of the EPM between groups (Figure 3D), indicating that the differences between groups cannot be explained by locomotor impairment.
Acoustic startle response
There was no difference in startle response or startle habituation between the groups (Figure 3F-G, respectively).
Retrospective classification and re-analyses by response pattern
Relative prevalence rates according to cutoff behavioral criteria: Significant differences were found between groups in the prevalence rates of animals displaying an EBR (Pearson χ2=10.27, df=2, p<0.006) (Figure 3H). Specifically, the prevalence of EBR was significantly higher in the blast-wave exposure group than in the unexposed (21.53% [14/65] and 6.25% [4/64], respectively; χ2=6.28, p<0.015) or sham-exposed groups (0% [0/22], χ2=5.16, p<0.025). There were no significant differences in the prevalence of MBR (control, 40% [26/64]; blast-exposed, 28% [18/65]; and sham-exposed, 20% [4/20]) (Figure 3I) or PBR (control, 53%, [34/64]; blast-exposed, 51% [33/65]; and sham-exposed, 80% [4/20]) (Figure 3J) between groups.
Assessment of spatial learning and memory
Swim speed averages were similar between groups. Rats from all three groups showed daily improvement in their learning and memory abilities.
Retrospective classification and re-analyses by response pattern
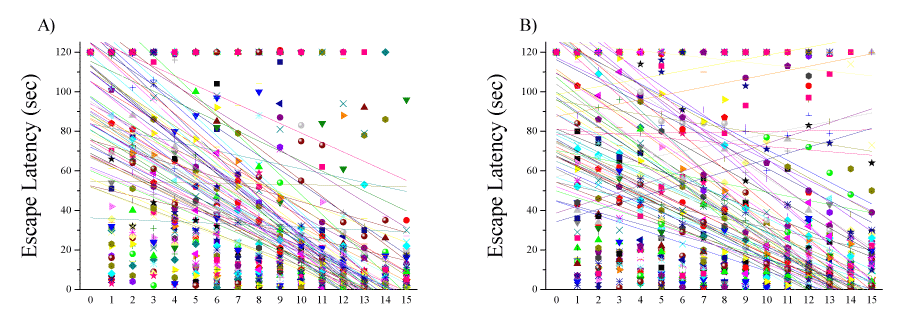
The exponential decay model was fitted to the escape latency (s) data in the acquisition (Figure 4A) and reversal (Figure 4B) phases in blast-exposed rats, and the prevalence rates of affected animals were calculated. We found that naïve middle-aged rats displayed very heterogeneous individual responses. Whereas some middle-aged rats performed as well as young rats, others showed pronounced cognitive deficits, and several were unable to find the platform at all. The variance made it complicated to determine "normal" criteria for learning patterns. Dynamic UFC was used to re-assess our results. The UFC analysis focused on the heterogeneous nature of the cognitive changes from baseline caused by a single blast-wave exposure in all rats. We set out to re-process the raw data to examine whether the UFC analysis would group animals in a similar manner to the cognitive grouping.
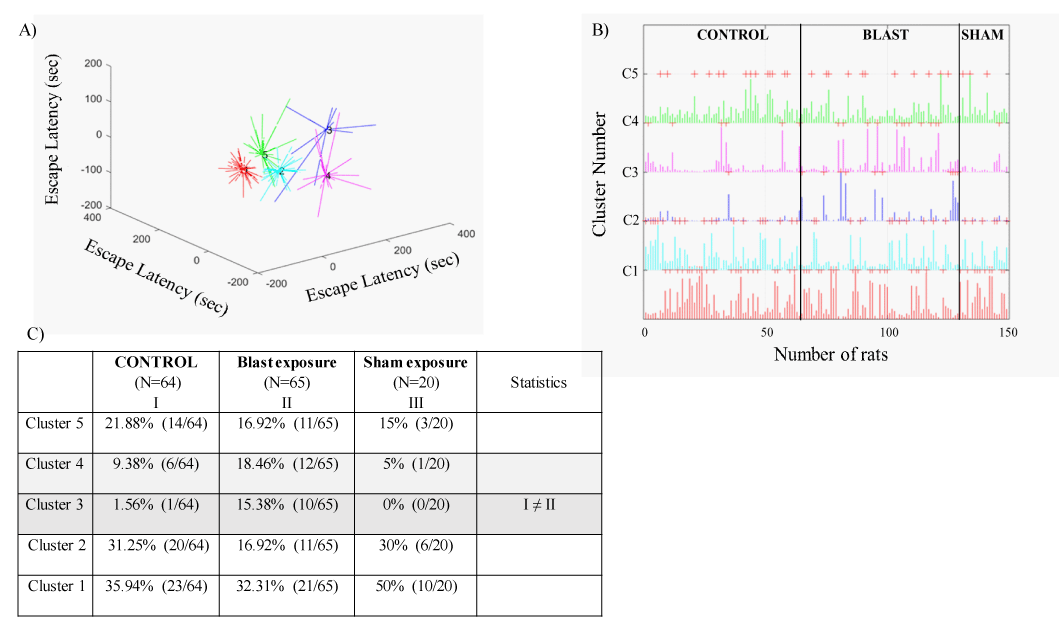
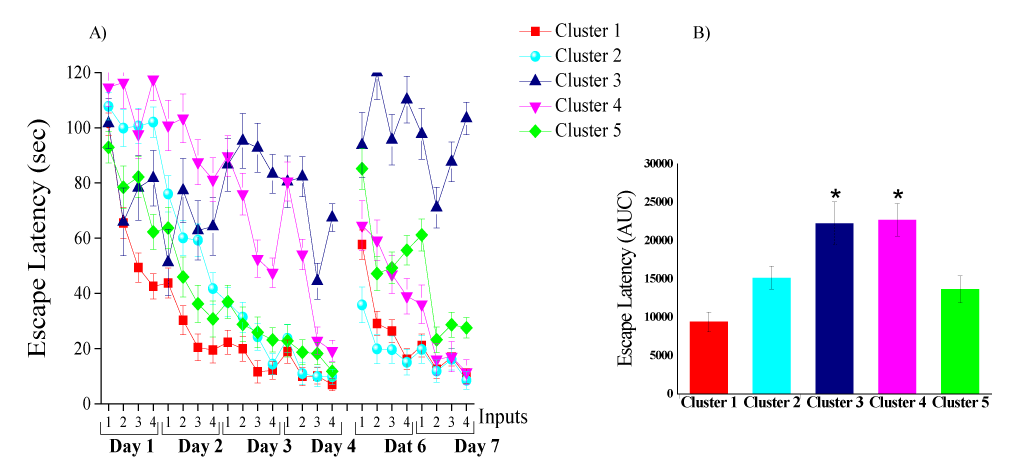
The UFC method was applied to sets of cognitive parametric data (escape latency in the acquisition and reversal phases) collected from 149 rats (64 controls, 65 blast-exposed, and 20 sham-exposed rats). The algorithm yielded five discrete clusters (C=5) for the dataset (Figure 5A-C). The centroid (mean clusters) of cluster 1 (C1) represents a short time spent swimming (spatial navigation) to reach the hidden platform for all days of acquisition and reversal. Thus, C1 describes a good and "normal" cognitive performance during the acquisition and reversal phases. The centroid of C2 represents a longer time spent swimming to reach the hidden platform during the acquisition phase as compared to the centroid of C1. Clusters 1 and 2 are not different regarding spatial navigation during the retrieval phase. Rats in C2 learned the task slowly, but once they learned, they performed the task properly. Rats in C5 showed the opposite learning pattern, followed by C2. The C5 centroid represents a longer time spent swimming to reach the hidden platform during the reversal phase as compared to the centroid of C1. Clusters 5 and 1 are not different regarding spatial navigation in the acquisition phase. Rats in C5 learned properly and performed poorly during the retrieval phase. The centroid of C3 represents a significant decline in spatial reference learning ability during the acquisition and reversal phases as compared to that in C1, C2, and C5. Rats associated with C3 completely lost their ability to learn, and thus C3 represents an extreme pathological response or TBI-like phenotype. The centroid of C4 represents a longer time spent swimming to reach the platform during both the acquisition and reversal phases as compared to the centroid of C1. Rats in C4 exhibited poor learning during the acquisition and phase. However, the rats in C4 used inefficient spatial navigation strategies and spent a longer time to find the hidden platform, but by the end of day 4 (during acquisition), they started to show normal learning patterns; thus, rats in C4 exhibited a mTBI-like phenotype.
In response to blast exposure, 32.3% (21/65) of rats were associated with C1, and 16.9% (11/65) of rats were associated with C2. In addition, 15.4% (10/65) of rats were associated with C3, 18.5% (12/65) were associated with C4, and 16.9% (11/65) were associated with C5.
In the unexposed control group, 36% (23/64) of the rats were associated with cluster C1, and 31.2% (20/64) were associated with C2. Only 1.56% (1/64) of the rats were associated with C3. In addition, 9.4% (6/64) of the rats were associated with C4, and 21.9% (14/64) were associated with C5.
In the sham-exposed group, 50% (10/20) of the rats were associated with C1, and 30% (6/20) were associated with C2. No rats were associated with C3. In addition, 5% (1/20) were associated with C4 and 15% (3/20) were associated with C5.
Significant differences in the prevalence rates of rats associated with different clusters (C1-C5) were found among the groups (Pearson χ2=18, df=8, p<0.025). The prevalence of rats associated with C3 was significantly higher in the blast-exposed group than in the unexposed group (15.38% [10/65] and 1.56% [1/64], respectively; χ2=8.04, p<0.005). In addition, the prevalence of rats associated with C3 was higher in the blast-exposed group than in sham-exposed rats (15.38% [10/65] and 0% [0/20], respectively; χ2=3.49, p=0.061). Most of the unexposed group (89%, 57/64) and sham-exposed group (95%, 19/20) were associated with clusters C1, C2, and C5, which indicated normal learning patterns in middle age. In addition, 9.4% (6/64) of the unexposed rats were associated with C4, the cluster that represents the mTBI-like phenotype. These rats show a pathological cognitive response that occurs with older age (Figure 6A-B).
Assessment of depression-related behavior
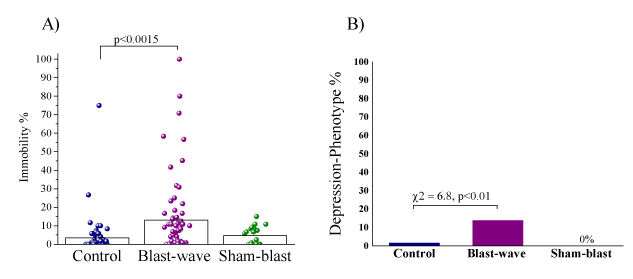
Significant differences were found between groups regarding immobility time during the forced swimming test (Figure 7A) (F[2,146]=7.23, p<0.0015). The immobility time during the swimming test was significantly prolonged in the blast-exposed group as compared with that in the control group (p<0.0015).
Retrospective classification and re-analyses by response pattern
Significant differences were found between groups in the prevalence rates of animals displaying a depressive phenotype (% immobility >30) (Pearson χ2=9.43, df=2, p<0.009) (Figure 7B). Specifically, the prevalence of a depressive phenotype was significantly higher in the blast-wave exposure group than in unexposed controls (13.85%, [9/65] and 1.56% [1/64]; χ2=6.8, p<0.01). The difference between the prevalence of the blast-exposed group and sham-exposed group (0%, [0/20]) trended towards significance (χ2=3.1, p=0.078).
Comparison among all affected groups
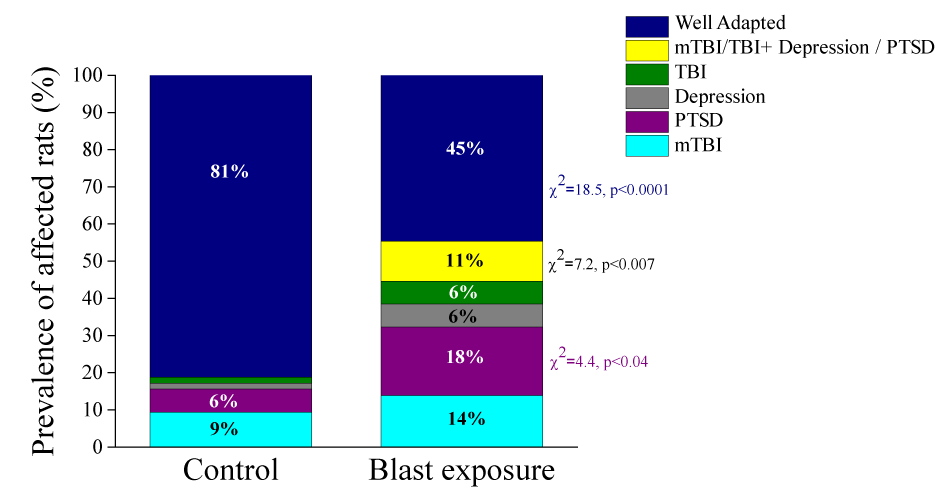
Due to the fact that the sham-exposed rats showed a well-adapted phenotype with no rats demonstrating an mTBI, PTSD, or depressive phenotype, we excluded that group from the prevalence results. Significant differences were found between groups in the prevalence rates of animals fulfilling our criteria for PTSD, mTBI-depression/PTSD, and WA (Figure 8). The prevalence of the WA-phenotype was significantly lower (χ2=18.52, p<0.0001) in the blast-wave exposure group than in the unexposed controls (44.620% [29/65] and 81.25% [52/64], respectively). The prevalence of the PTSD-phenotype was significantly higher (χ2=4.43, p<0.04) in the blast-wave exposure group than in the unexposed controls (18.46% [12/65] and 6.25% [4/64], respectively). The prevalence of mTBI-depression/PTSD phenotype was significantly higher (χ2=7.29, p<0.007) in the blast-wave exposure group than in the unexposed controls (10.77% [7/65] and 0% [0/64], respectively). There were no significant differences in the prevalence of the depressive phenotype between the blast-exposed and control groups (6.15% [4/65] and 1.56% [1/64], respectively). There were no significant differences in the prevalence of the mTBI-PTSD phenotype between the blast-exposed and control groups (13.85% [9/65] and 9.38% [6/64], respectively). There were no significant differences in the prevalence of the TBI-PTSD-phenotype between the blast-exposed and control groups (6.15% [4/65] and 1.56% [1/64], respectively).
Discussion
The response patterns of the blast-wave exposure model employed in this study replicate our previous data [9] in that they demonstrated that exposure to a single low-pressure blast-wave can produce distinctive long-lasting psycho-neurobehavioral responses that model PTSD, mTBI, and comorbid PTSD-mTBI sequelae in a significant proportion of animals. However, middle-aged rats displayed a different pattern of behavioral outcomes following blast exposure as compared to our previous results in young rats [4,5,25]. This finding was especially evident for depression-related behavior and in a significant decline in spatial reference learning ability, findings not observed at all in younger rats. Moreover, we found that exposure to the ‘‘flash-bang’’ sensory experience of the experimental detonation (under sham conditions without blast-wave exposure) elicited only minor behavioral and cognitive responses. Only rats exposed to the full experimental conditions developed severe behavioral and cognitive response changes, whereas none of the sham-exposed rats fulfilled the criteria for the PTSD phenotype, depressive phenotype, or mTBI phenotype. Taken together, these results indicate that middle-aged rats respond differently to blast exposure than young rats and that age is an important factor to consider in pre-clinical efficacy studies.
PTSD-like phenotype
The results demonstrate that exposure to the ‘‘flash-bang’’ sensory experience of the experimental detonation in middle-aged rats, whether under sham conditions (without blast-wave) or in the face of the blast-wave, aroused significant anxiety- and avoidance-related behaviors in the EPM relative to unexposed controls. The lack of significant differences between groups in the mean startle amplitude in the ASR paradigm may have stemmed from a “ceiling effect”, as it was relatively high in all groups (compared, for instance, to our previous study in younger rats). Application of the criteria designed for traumatic stress studies to the data revealed that between those two groups, only rats exposed to the full experimental conditions (i.e., blast-wave exposure) developed severe behavioral response patterns (with a prevalence of 18% for extreme behavioral responders), whereas none of the sham-exposed group fulfilled criteria for EBR (PTSD like phenotype). This finding suggests that only direct exposure to the experimental detonation caused PTSD-like behavioral responses; that is, extreme and long-term behavioral disruption, whereas sham exposure elicited only short-term anxiety-like responses. Moreover, the data clearly demonstrate that only a minority of fully exposed rats developed long-term behavioral disruption, whereas the majority displayed minor or no long-term disruption to low-pressure blast exposure, consistent with the investigators’ intentions. This result shows the same behavioral pattern observed in young rats [4]. We found two main differences between young and middle-aged ratsUnlike young rats, middle-aged rats exposed to sham conditions remained in the central EPM platform longer. Rats invariably prefer locations within the closed arms, avoiding the unprotected open arms due to fear-related anxiety associated with exposure plus the elevation of the apparatus above the floor. The longer time spent in the central platform is counterbalanced by a reduction in the activity in the open arms of the EPM, as demonstrated by the significant reduction in the mean frequency of various “unprotected” elements. Therefore, it has been reported that the time spent in this zone of the maze is linked to decision-making processes triggered by the strong approach/avoidance conflict induced by the apparatus [26-28].
Among middle-aged rats, behavioral performance was impaired even without blast-wave exposure. We found that 6.25% of the middle-aged rats in the unexposed control group exhibited a PTSD-like phenotype. The elevated anxiety and PTSD observed in middle-aged rats is consistent with the greater anxiety response reported in older animals [29]. This phenotype was not observed in young rats and could be indicative of the influence of age on behavioral and cognitive aspects.
TBI and mTBI-like phenotype
Naïve middle-aged rats displayed heterogeneous individual responses. Whereas some middle-aged rats performed as well as young rats, others showed pronounced cognitive deficits, and several were unable to find the platform at all. However, no age-related deficits in motor coordination, swimming efficiency, or spontaneous locomotion and exploration were observed. It is well established that aging leads to a progressive loss of cognitive function, especially in spatial memory. Most reports indicate that aged rats, as a group, are impaired in comparison with young rats on both the working and reference memory components of spatial tasks [30-33]. One of the hallmarks of research on age-related spatial memory impairment, however, is that groups of aged rats show a broader range of variability than groups of young rats on measures of spatial memory and on measures of relevant neurobiological markers [34-36]. Thus, some aged rats are impaired on spatial memory tasks, whereas other aged rats perform as well as young rats. The variance made it complicated to determine "normal" criteria for learning patterns. Therefore, in order to re-assess the results, we used dynamic UFC [16,37].
In this study, the UFC model partitioned cognitive parameters (escape latency in the acquisition and reversal phases) into five clusters. The first cluster (C1) is related to "normal" cognitive performance and is characterized by a high level of cognitive performance in the spatial learning and memory task. This cluster shows an exponential pattern of spatial navigation and learning, which indicates that these rats learn to find the hidden platform rapidly, as we reported in young rats [4]. The second and fifth clusters (C2 and C5) are characterized by some degree of slow performance. There seems to be a general slowdown pattern in the performance in both clusters; however, each cluster exhibits slow learning in a different part of the learning, acquisition, and retrieval phases. Rats in C2 demonstrated slow performance during the first two days of the acquisition phase. It took them a much longer time to find the hidden platform (to learn), but once they learned, they succeeded in all other phases. Rats in C5 exhibited the opposite performance pattern. They learned to find the hidden platform quickly and showed great success in the first 4 days of the acquisition phase, but once the platform was moved (during the retrieval phase), their performance was severely impaired. One may speculate that these three clusters (C1, C2, and C5) represent the “normal” learning ability in middle age, in which naïve middle-aged rats displayed very heterogeneous individual responses. Based on the escape latency features of all three clusters, it is quite clear that most of the control animals (90.0% in the unexposed group and 95.0% in the sham-exposed group) were associated with these clusters, and differed significantly from those in the blast-exposed group (66.15%) (χ2=9.71, p<0.002 and χ2=6.45, p< 0.015, respectively). These response patterns replicate our previous data [9] in that they demonstrated that most of the exposed animals did not demonstrate any behavioral or cognitive abnormalities. C4 represents the subclinical or partially affected animals and exhibits an mTBI-like cognitive phenotype. These mild deficits in spatial learning and memory tasks were also observed in control rats. In the control group, six rats (9.38%) in the unexposed group and one rat (5%) in the sham-exposed group, were associated with this cluster, whereas 12 rats from the blast group (18.46%) were associated with this cluster (C4). Thus, these mild deficits in spatial learning and memory are associated with both age-related cognitive decline and blast-related pathology. C3 represents the significantly clinically affected animals and is characterized by higher levels of learning and memory disorder, namely the extreme TBI-phenotype from the blast explosion group. Rats in this cluster exhibited a significant decline in spatial learning and memory ability, without any significant improvement. In fact, rats in this cluster did not demonstrate learning at all in either the acquisition phase or in the reversal phase. In the control group, only one rat (1.56%) was associated with cluster C3, whereas 10 rats from the blast-exposed group (15.37%) were associated with this cluster. All rats that exhibited significant deficits in the MWM showed no problems in the EPM and ASR paradigms or in the NSS, thus reducing the possibility that the performance in the learning and memory task was due to problems with vision, motor ability, or motivation. This extreme response group (i.e., the TBI-like phenotype) exhibited significantly more pronounced cognitive deficits than other animals. Moreover, these deficits in spatial learning and memory were not shown in our previous study using young rats [4]. Taken together, it may be that this UFC model of clustering has succeeded in accounting for the “variance noise” and is able to show the unique contribution of blast-wave exposure and aging.
These results are in agreement with the previous well-established findings reported that both mice and rats exhibit deficits in spatial memory performance during aging [38-42]. Hoane, et al. [43] reported that following frontal cortex contusions, middle-aged (14 months) rats performed worse on a variety of behavioral tests than young rats. The authors reported that middle-aged rats were significantly more impaired than young rats in their ability to acquire a reference memory task in the MWM [43]. Moreover, the middle-aged brain was more vulnerable to injury than the younger brain, and the middle-aged rats developed significantly larger lesion cavities compared to young rats [43]. An earlier study by Hamm, et al. [44] reported that 20-month-old Fischer 344 rats had a higher mortality rate and greater incidence of acute neurological deficits than young adult animals following both low (1.7 to 1.8 atm) and moderate (2.00 to 2.25 atm) brain injuries using the fluid percussion model. The Fischer 344 rat strain was chosen in Hamm's study because it does not continue to grow throughout its life span; therefore, adult rats of different ages are not vastly different in weight, an important factor when inducing a traumatic brain injury in rodents. Moreover, 20-month-old rats with fluid percussion injury showed greater impairments in beam-walking, a motor task, and in the acquisition of a reference memory in the MWM [45].
Depression-like phenotype
The results demonstrated that exposure to low-pressure blast-waves in middle age significantly increased the prevalence of depression-like behavior. This phenotype was very surprising since in young rats this phenotype was not seen at all. Even rats that showed a stress-related phenotype did not show depressive-like behavior. This finding is crucial because it may demonstrate one of the unique differences between reacting to blast-waves at a young age and in middle age.
Studies of the association between aging and depression-related behavior in rats and mice have been inconclusive, with some studies reporting a positive association [46-52] and others no association [49,53,54].
Clinically, no distinction is made in the DSM-5 depression criteria based on age or aging [55]. Casey et al. [55] reported that the prevalence of depression in older American adults appears to be similar to that in other age groups. Moreover, qualitative meta-analyses of both cross-sectional and longitudinal studies showed that older age appears to be an important risk factor for depression in the general elderly population (aged below 80 years), but not in the oldest population (aged above 85 years) [56].
Comparison among all affected groups
Overall, the results of the model employed in this study support the idea that exposure to a single low-pressure blast-wave can produce long-lasting neurobehavioral sequelae. The results demonstrate a degree of heterogeneity in individual responses to the experimental blast-wave. Although a considerable percentage of the exposed animals did not demonstrate any behavioral or cognitive abnormalities, exposure to the experimental blast-wave did elicit TBI - and mTBI-like behavior, a PTSD-like phenotype, depression-like symptoms, and combined mTBI/TBI-depression/PTSD-like symptoms. In general, the prevalence of the WA-phenotype was significantly lower (45%) in the blast-wave exposure group than in the unexposed controls (81%, χ2=18.52, p<0.0001). This difference is significant and shows that the majority of both groups were not affected, but still in the blast group, 45% of animals were affected. The classification and diagnosis of the different diseases was difficult due to the major heterogeneity that was discussed above. Nevertheless, we found more PTSD-like phenotypes in middle-aged rats, a TBI-phenotype, and a depressive-like phenotype that was not found in young rats.
In a clinical study, in contrast to our results, Arbour, et al. [57] showed that age did not have a negative effect on the potential for functional recovery and resiliency after brain trauma in adults of working age. Additionally, in a meta-analysis of 57 studies that reported on the prevalence of depression in a population with mild cognitive impairment, representing 20,892 participants, the overall prevalence of depression was 32% [58]. The authors found that there were differences in prevalence estimates between community samples (25%) and clinical samples (40%). However, the relationships between mTBI/TBI, depression, and age are complicated and complex. Aging and TBI independently are known to be causal/risk factors for depression, and elderly individuals represent a population vulnerable to both TBI and depression.
Conclusions
Overall, the study emphasizes the complexity of working with older subjects, both in terms of determining "baseline norms" and in terms of the pattern of responses to the paradigm. These characteristics are also found with regard to studies involving older human subjects, certainly in terms of differing clinical presentations compared to younger populations, but also in terms of age-related baseline characteristics.
Our results support the idea that exposure to a single low-pressure blast-wave can produce long-lasting psychoneurobehavioral sequelae. The results demonstrated a satisfactory degree of heterogeneity in the animals’ responses to experimental blast exposure. Exposure to an experimental blast-wave elicited distinct behavioral responses modeling depressive-like phenotype, mTBI-like phenotype, PTSD-like phenotype, and comorbid mTBI-PTSD-like phenotype responses. The middle-aged rats displayed significantly worse behavioral outcomes following blast exposure than young rats. This finding was especially evident in depression-related behavior and a significant decline in spatial reference learning ability, which was not observed in younger rats. These results indicate that middle-aged rats respond differently to blast exposure than young rats and that age is an important factor to consider in pre-clinical efficacy studies.
- Xydakis MS, Butman JA, Pierpaoli C (2011) Blast-related traumatic brain injury in U.S. military personnel. N Engl J Med 365: 859. Link: https://bit.ly/3nOW848
- Wolf SJ, Bebarta VS, Bonnett CJ, Pons PT, Cantrill SV (2009) Blast injuries. Lancet 374: 405-415. Link: https://bit.ly/373VnO9
- Wang C, Pahk JB, Balaban CD, Miller MC, Wood AR, et al. (2014) Computational study of human head response to primary blast waves of five levels from three directions. PLoS One 9: e113264. Link: https://bit.ly/339x2Fl
- Zuckerman A, Ram O, Ifergane G, Matar MA, Sagi R, et al. (2017) Controlled Low-Pressure Blast-Wave Exposure Causes Distinct Behavioral and Morphological Responses Modelling Mild Traumatic Brain Injury, Post-Traumatic Stress Disorder, and Comorbid Mild Traumatic Brain Injury-Post-Traumatic Stress Disorder. J Neurotrauma 34: 145-164. Link: https://bit.ly/3pRORSW
- Zuckerman A, Ram O, Ifergane G, Matar MA, Kaplan Z, et al. (2019) Role of Endogenous and Exogenous Corticosterone on Behavioral and Cognitive Responses to Low-Pressure Blast Wave Exposure. J Neurotrauma 36: 380-394. Link: https://bit.ly/36XnaQ4
- Sun M, Brady RD, Casillas-Espinosa PM, Wright DK, Semple BD, et al. (2019) Aged rats have an altered immune response and worse outcomes after traumatic brain injury. Brain Behav Immun 80: 536-550. Link: https://bit.ly/37f7B6H
- Hukkelhoven CW, Steyerberg EW, Rampen AJ, Farace E, Habbema JD, et al. (2003) Patient age and outcome following severe traumatic brain injury: an analysis of 5600 patients. J Neurosurg 99: 666-673. Link: https://bit.ly/3m8u8rG
- Susman M, DiRusso SM, Sullivan T, Risucci D, Nealon P, et al. (2002) Traumatic brain injury in the elderly: increased mortality and worse functional outcome at discharge despite lower injury severity. J Trauma 53: 219-223. Link: https://bit.ly/3fn7GZo
- Zuckerman A, Ram O, Ifergane G, Matar MA, Sagi R, et al. (2016) Controlled Low-Pressure Blast-Wave Exposure Causes Distinct Behavioral and Morphological Responses Modelling Mild Traumatic Brain Injury, Post-Traumatic Stress Disorder, and Comorbid Mild Traumatic Brain Injury-Post-Traumatic Stress Disorder. J Neurotrauma 34: 145-164 Link: https://bit.ly/3fnM27r
- Slotkin TA, Cousins MM, Tate CA, Seidler FJ (2005) Serotonergic cell signaling in an animal model of aging and depression: olfactory bulbectomy elicits different adaptations in brain regions of young adult vs aging rats. Neuropsychopharmacology 30: 52-57. Link: https://bit.ly/3nLnRCU
- Ram O, Sadot O (2012) Implementation of the exploding wire technique to study blast-wave-structure interaction. Exp Fluids 53: 1335-1345. Link: https://bit.ly/2UQRSVp
- Ram O, Nof E, Sadot O (2016) Dependence of the blast load penetrating into a structure on initial conditions and internal geometry. Experimental Thermal and Fluid Science 78: 65-74. Link: https://bit.ly/3nOvngs
- Ram O, Sadot O (2013) A simple constitutive model for predicting the pressure histories developed behind rigid porous media impinged by shock waves. Journal of Fluid Mechanics 718: 507-523. Link: https://bit.ly/3nMo2h7
- Feldman Z, Gurevitch B, Artru AA, Oppenheim A, Shohami E, et al. (1996) Effect of magnesium given 1 hour after head trauma on brain edema and neurological outcome. J Neurosurg 85: 131-137. Link: https://bit.ly/3pRPLii
- Cohen H, Matar MA, Joseph Z (2013) Animal models of post-traumatic stress disorder. Curr Protoc Neurosci. Link: https://bit.ly/3nOvqc8
- Cohen H, Zohar J, Matar MA, Kaplan Z, Geva AB (2005) Unsupervised fuzzy clustering analysis supports behavioral cutoff criteria in an animal model of posttraumatic stress disorder. Biol Psychiatry 58: 640-650. Link: https://bit.ly/3lR62lg
- Cohen H, Zohar J, Matar MA, Zeev K, Loewenthal U, et al. (2004) Setting apart the affected: the use of behavioral criteria in animal models of post traumatic stress disorder. Neuropsychopharmacology 29: 1962-1970. Link: https://bit.ly/372k5Oz
- Cohen H, Zohar J (2004) An animal model of posttraumatic stress disorder: the use of cut-off behavioral criteria. Ann N Y Acad Sci 1032: 167-178. Link: https://bit.ly/33acf4y
- Cohen H, Zohar J, Matar M (2003) The relevance of differential response to trauma in an animal model of posttraumatic stress disorder. Biol Psychiatry 53: 463-473. Link: https://bit.ly/395gD8z
- Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11: 47-60. Link: https://bit.ly/373aGX0
- Doppenberg EM, Choi SC, Bullock R (2004) Clinical trials in traumatic brain injury: lessons for the future. J Neurosurg Anesthesiol 16: 87-94. Link: https://bit.ly/2UQI8uf
- Jaffee M, Helmick K, Girard P, Meyer K, Dinegar K, et al. (2009) Acute clinical care and care coordination for traumatic brain injury within Department of Defense. J Rehabil Res Dev 46: 655-666. Link: https://bit.ly/3kW61vh
- Geva AB (1998) Feature extraction and state identification in biomedical signals using hierarchical fuzzy clustering. Medical & Biological Engineering & Computing 36: 608-614. Link: https://bit.ly/2Hr83FX
- Geva AB, Pratt H (1994) Unsupervised clustering of evoked potentials by waveform. Med Biol Eng Comput 32: 543-550. Link: https://bit.ly/36XyR9v
- Hoffman JR, Zuckerman A, Ram O, Sadot O, Stout JR, et al. (2017) Behavioral and inflammatory response in animals exposed to a low-pressure blast wave and supplemented with beta-alanine. Amino acids 49: 871-886. Link: https://bit.ly/2UOkDC8
- Cruz AP, Frei F, Graeff FG (1994) Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav 49: 171-176. Link: https://bit.ly/3pMbmbY
- Rodgers RJ, Johnson NJ (1995) Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol Biochem Behav 52: 297-303. Link: https://bit.ly/2IYqimI
- Rodgers RJ, Lee C, Shepherd JK (1992) Effects of diazepam on behavioural and antinociceptive responses to the elevated plus-maze in male mice depend upon treatment regimen and prior maze experience. Psychopharmacology (Berl) 106: 102-110. Link: https://bit.ly/3m0N7oc
- Shoji H, Mizoguchi K (2010) Acute and repeated stress differentially regulates behavioral, endocrine, neural parameters relevant to emotional and stress response in young and aged rats. Behav Brain Res 211: 169-177. Link: https://bit.ly/3lR0g37
- Ando S, Ohashi Y (1991) Longitudinal study on age-related changes of working and reference memory in the rat. Neuroscience Letters 128: 17-20. Link: https://bit.ly/370FFD7
- Jucker M, Oettinger R, Bättig K (1988) Age-related changes in working and reference memory performance and locomotor activity in the Wistar rat. Behavioral and Neural Biology 50: 24-36. Link: https://bit.ly/36ZD9gz
- Pitsikas N, Algeri S (1992) Deterioration of spatial and nonspatial reference and working memory in aged rats: protective effect of life-long calorie restriction. Neurobiology Agiong 13: 369-373. Link: https://bit.ly/3nSslI1
- van der Staay FJ, de Jonge M (1993) Effects of age on water escape behavior and on repeated acquisition in rats. Behavioral and Neural Biology 60: 33-41. Link: https://bit.ly/36YYpDc
- Gage FH, Chen KS, Buzsaki G, Armstrong D (1988) Experimental approaches to age-related cognitive impairments. Neurobiology of Aging 9: 645-655. Link: https://bit.ly/338Xy1M
- Gallagher M, Nicolle MM (1993) Animal models of normal aging: relationship between cognitive decline and markers in hippocampal circuitry. Behav Brain Res 57: 155-162. Link: https://bit.ly/3nKWgle
- Gallagher M, Burwell RD (1989) Relationship of age-related decline across several behavioral domains. Neurobiology Aging 10: 691-708. Link: https://bit.ly/33967cV
- Cohen H, Geva AB, Matar MA, Zohar J, Kaplan Z (2008) Post-traumatic stress behavioural responses in inbred mouse strains: can genetic predisposition explain phenotypic vulnerability? Int J Neuropsychopharmacol 11: 331-349. Link: https://bit.ly/2KyYW7l
- Magnusson KR, Scruggs B, Zhao X, Hammersmark R (2007) Age-related declines in a two-day reference memory task are associated with changes in NMDA receptor subunits in mice. BMC Neurosci 8: 43. Link: https://bit.ly/3pMT7TX
- Barnes CA (1988) Aging and the physiology of spatial memory. Neurobiology Aging 9: 563-568. Link: https://bit.ly/2IZAZoQ
- Gage FH, Dunnett SB, Björklund A (1984) Spatial learning and motor deficits in aged rats. Neurobiol Aging 5: 43-48. Link: https://bit.ly/3pS3DJA
- D'Hooge R, De Deyn PP (2001) Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev 36: 60-90. Link: https://bit.ly/2IQoIDF
- Brandeis R, Brandys Y, Yehuda S (1989) The use of the Morris Water Maze in the study of memory and learning. Int J Neurosci 48: 29-69. Link: https://bit.ly/3lWHKXb
- Hoane MR, Lasley LA, Akstulewicz SL (2004) Middle age increases tissue vulnerability and impairs sensorimotor and cognitive recovery following traumatic brain injury in the rat. Behav Brain Res 153: 189-197. Link: https://bit.ly/2UNQ8MK
- Hamm RJ, Jenkins LW, Lyeth BG, White-Gbadebo DM, Hayes RL (1991) The effect of age on outcome following traumatic brain injury in rats. J Neurosurg 75: 916-921. Link: https://bit.ly/2IZBtva
- Hamm RJ, White-Gbadebo DM, Lyeth BG, Jenkins LW, Hayes RL (1992) The effect of age on motor and cognitive deficits after traumatic brain injury in rats. Neurosurgery 31: 1072-1077. Link: https://bit.ly/2IRivHC
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, et al. (2008) Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology 33: 2341-2351. Link: https://bit.ly/2URz9Jo
- Ohashi S, Mori A, Kurihara N, Mitsumoto Y, Nakai M (2006) Age-related severity of dopaminergic neurodegeneration to MPTP neurotoxicity causes motor dysfunction in C57BL/6 mice. Neurosci Lett 401: 183-187. Link: https://bit.ly/2UR0taH
- Shoji H, Takao K, Hattori S, Miyakawa T (2016) Age-related changes in behavior in C57BL/6J mice from young adulthood to middle age. Molecular brain 9: 11. Link: https://bit.ly/2KtOpKt
- Malatynska E, Steinbusch HW, Redkozubova O, Bolkunov A, Kubatiev A, et al. (2012) Anhedonic-like traits and lack of affective deficits in 18-month-old C57BL/6 mice: Implications for modeling elderly depression. Exp Gerontol 47: 552-564. Link: https://bit.ly/3739LpN
- Moretti M, de Souza AG, de Chaves G, de Andrade VM, Romao PR, et al. (2011) Emotional behavior in middle-aged rats: Implications for geriatric psychopathologies. Physiology & Behavior 102: 115-120. Link: https://bit.ly/3lVQEUP
- Shoji H, Miyakawa T (2019) Age-related behavioral changes from young to old age in male mice of a C57BL/6J strain maintained under a genetic stability program. Neuropsychopharmacology Rep 39: 100-118. Link: https://bit.ly/2UOaBkq
- Frye CA, Edinger KL, Lephart ED, Walf AA (2010) 3alpha-androstanediol, but not testosterone, attenuates age-related decrements in cognitive, anxiety, and depressive behavior of male rats. Front Aging Neurosci 2: 15. Link: https://bit.ly/2V4ENrT
- David DJ, Bourin M, Hascoët M, Colombel MC, Baker GB, et al. (2001) Comparison of antidepressant activity in 4- and 40-week-old male mice in the forced swimming test: involvement of 5-HT1A and 5-HT1B receptors in old mice. Psychopharmacology (Berl) 153: 443-449. Link: https://bit.ly/3kWQ5c7
- Kasckow JW, Segar TM, Xiao C, Furay AR, Evanson NK, et al. (2005) Stability of neuroendocrine and behavioral responsiveness in aging Fischer 344/Brown-Norway hybrid rats. Endocrinology 146: 3105-3112. Link: https://bit.ly/2UQY5Rb
- Casey DA (2017) Depression in Older Adults: A Treatable Medical Condition. Prim Care 44: 499-510. Link: https://bit.ly/3lR1prr
- Zhao KX, Huang CQ, Xiao Q, Gao Y, Liu QX, et al. (2012) Age and risk for depression among the elderly: a meta-analysis of the published literature. CNS Spectr 17: 142-154. Link: https://bit.ly/35VaYjG
- Arbour C, Gosselin N, Levert MJ, Gauvin-Lepage J, Michallet B, et al. (2017) Does age matter? A mixed methods study examining determinants of good recovery and resilience in young and middle-aged adults following moderate-to-severe traumatic brain injury. J Adv Nurs 73: 3133-3143. Link: https://bit.ly/36ZE7JJ
- Ismail Z, Elbayoumi H, Fischer CE, Hogan DB, Millikin CP, et al. (2017) Prevalence of Depression in Patients With Mild Cognitive Impairment: A Systematic Review and Meta-analysis. JAMA Psychiatry 74: 58-67. Link: https://bit.ly/3pRLuvp
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
