Archives of Depression and Anxiety
Influence of social isolation and aggressive behavior in the appearance of Depression-like in Experimental model
Gabriel Melo de Oliveira* and Cleiton Felizardo Brito
Cite this as
de Oliveira GM, Brito CF (2019) Influence of social isolation and aggressive behavior in the appearance of Depression-like in Experimental model. Arch Depress Anxiety 5(2): 042-046. DOI: 10.17352/2455-5460.000040The study of animal behavior in the laboratory environment aims to promote welfare and minimize the discomfort of animals during scientific tests. This knowledge is important to understand social interactions and develop methods of environmental enrichment, thus increasing the quality of life of animals housed in the laboratory. The goal this study was evaluated the individual and social behavior and influence in the anxiety or depression-like condition in mouse during aggressive behavior and compared with social isolation in the animal facilities. Observing their daily routine, the presence of aggressive behavior patterns of individual mice, mainly male adult mouse. We used two ethological methods: i) Open-field and ii) Elevated plus-maze test in the three categories of behavior observed, dominant, subordinate and isolated mice. Our results showed that in groups where there was the presence of aggressive behavior patterns (in both ethological tests) motor and exploratory activity in dominant and subordinate categories was inferior when compared with to harmonic interaction group. Furthermore, subordinate and isolated mice showed a significant decrease in activity, compatible with depressive behavior described in the literature for experimental models. Concluded, from these results we were able to observe that social disturbance and aggressive behavior during social and individual interaction promoted discomfort and stress in mice lab and depression-like state.
Introduction
Actually, there is intense discussion related to the laboratory animal welfare in biomedical testing. Ethical parameters in the use of laboratory animals are, mainly, based in the 3Rs principles [1]. Briefly, these principles suggest: i) substitution of animals by alternative methods; ii) reduction in the number of animals used and iii) refinement of housing and manipulation techniques. In non-human primate model various studies have addressed the question of promoting an environment for individuals to develop their exploratory activity and increase the quality of social interaction [2]. Accordingly, studies recommend trying to find better techniques for the maintenance of laboratory animals to promote the welfare of individuals and minimize animal discomfort [2,3]. For rodents there are also several studies related to environmental enrichment [4,5]. However, indexed literature analysis described (Pub Med - August 2019) a relatively small amount of research into the individual and social behavior of mice. About 49,763 studies have evaluated the issue of welfare in non-human primates, whereas only 316 corresponding works related on mice [6]. Hence, the perceived necessity for the study of mice behavior in the laboratory in order to promote knowledge that could be used in the development of new equipment, procedures and techniques, improving the welfare of laboratory mice. Furthermore, we observe that this knowledge in the animal behavior is also important to improve the empathy of the manipulator in relation to the animal, increasing the quality in the procedures performed and minimizing the occurrence of biological accidents [6].
Territorialim, sexual selection and social interaction was intrinsic characteristic of the mouse lab and dominant behavior can be observed in both male and female mice. In the adult male mice, the aggressive behavior, there is high prevalence of behavior between adult individuals. The appearance of these fights is easily observable during routine cleaning and handling of these animals [7]. The aggression between male mice may have several causes: genetic [8], hormonal [9,10] or neurochemical [11,12] among others. It´s suggested that this aggression could be related to the struggle of individuals for social domination within the structural hierarchy [13]. However, some studies suggest that the presence of aggression occurs because of the failure of the harmonic hierarchy [14]. The presence of dominant and subordinate (attacked) individuals was observed in several male groups. We can identify mice that promote aggression and others who suffer attacks [15]. It is likely that mice suffering attacks are those that are in an inferior position in social dominance [16]. The social isolation of the aggressor animal is the main attitude in the management of aggressive animals [16].
The aim this study is to investigated the influence of the aggressive behavior in emotional state of the mice lab during housing in the animal facilities. We use motor and exploratory activity and pattern of aggressive behavior (PAB) in the all groups (and categories), and social isolation animal. Using ethological methods, normally employed for the evaluation of animal models of depression and anxiety [16], we measured the level of motor and exploratory activity in dominant, subordinate and isolated mice [17]. Then our proposal was to compare among the categories studied the presence of the state of anxiety and depression, mainly in the management of social isolation and in a situation of exacerbated aggression in groups of mice lab. Animals maintained in the absence of social interaction, described in the literature as a model for hypoactivity or depression-like behavior, were used as a reference [18,19].
Material and Methods
Mice
Male Swiss Webster mice were obtained from Instituto de Ciência e Tecnologia em Biomodelos (ICTB/Fiocruz) and maintained at LAboratory Animal Science Division of the Biotério de Experimentação Animal (LBC-LITEB – Instituto Oswaldo Cruz). The animals were adapted to the environment for one week in ventilated racks, and the temperature, humidity and photoperiod were controlled according to the standard environmental regulations. The animals were maintained under stable conditions of temperature and light, with a 12-h light/dark cycle, and both food and water were available ad libitum. The animals were male, adult Swiss Webster mice (over ten weeks of life). This project has license number (L – 10/18) at the Animal Ethical Use CEUA - IOC/FIOCRUZ. We used a total number of 3 to 4 animals per triplicate assay.
Pattern of aggressive behavior (PAB)
We evaluated the daily routine, looking for the appearance of signs of aggression, fights, bites and dominance in each animal group. We also noted other stress signs such as vocalizations, weight loss and others. Thus identifying and categorizing the groups and individuals who showed patterns of aggressive behavior.
Animal behavior categories: We divided the animals into four categories: i) Normal (norm), where none of the individuals had PAB in group. ii) Dominant (dnte), characterized by individuals in groups with PBA presence. iii) Subordinate (subt), related to animals that had bites and injuries in groups with PAB. iv) Solitary isolation (solit), mice that were housed without social interaction.
Open field test
This test consisted of the measurement of behavioral variables in experimental individuals, placed in an arena limited to sixteen spaces of equal size [20]. Behavioral assessment was carried out by evaluating motor activity by measuring the number of spaces crossed (horizontal displacements) by each mouse in a period of five minutes, while the exploratory activity was measured through the number of vertical displacements (rearing) independent of the location in the arena, also in a period of five minutes.
Elevated plus maze test
This test was used to assess the motor ability and exploratory interest of each animal [21]. The assessment of exploratory interest and locomotor ability was carried out through the measurement of the entry into and time spent by the individual mice in the closed and open arms over a period of five minutes.
Statistical analysis
Results were expressed as mean-standard deviation (±SD). Significance between means was determined using the Student t-test and results were regarded as significant when P ≤ 0.05.
Results
Mice lab observation and PAB and isolation social behavior in their daily routine we divided them into respective categories. The behavior types was demonstrated graphically (Figure 1). The first category described is of mice that did not demonstrate aggressive behavior in the group, denominated as normal (Figure 1A). In relation to PAB groups we distinguished two categories of individual aggressive behavior. Dominant animals were those who showed no signs of fights or bites. Subordinate animals, in turn, showed signs of fights and bites. In these groups, two types of social dominance was suggested: i) «one against all» when an individual fought all components (Figure 1B) and ii) «all against one» when several components fought only one individual component of the group (Figure C), but we have not yet confirmed this dynamic of aggressiveness. Moreover, we also evaluated the activity of mice in social isolation (Figure 1D).
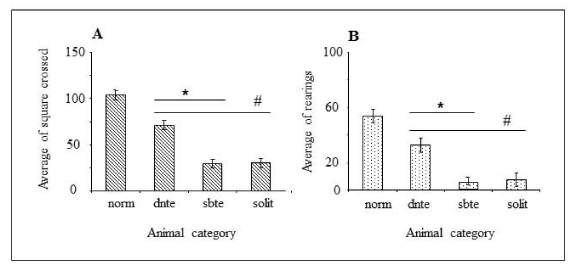
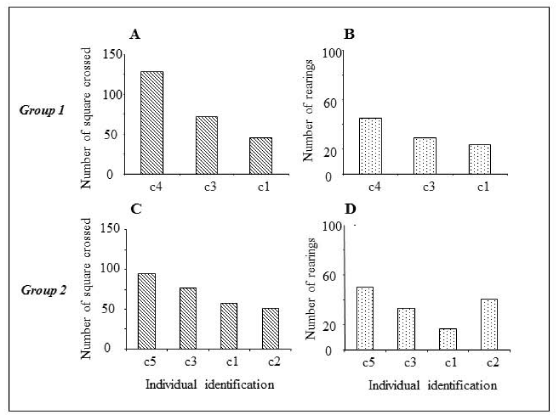
Open field test results (Figure 2) showed that both, dominant and subordinate mice, showed a significant decrease in motor (Figure 2A) and exploratory activity (Figure 2B) when compared to normal mice. Subordinate animals demonstrated lower activity when compared with the dominant mice and a similar level in relation to the animals in social isolation (Figure 2A,B) both in terms of horizontal and vertical displacement. Individual physical activity of the dominant and subordinated was evaluated of the two concomitants groups (Figure 3). The results demonstrated that each mice showed individual value of motor and exploratory activity. Interestingly, we observed a decreasing level in the individual profile of horizontal (Figure 3A-C) and vertical (Figure 3B-D) displacements of the mice in both cages, mainly subordinated mice.
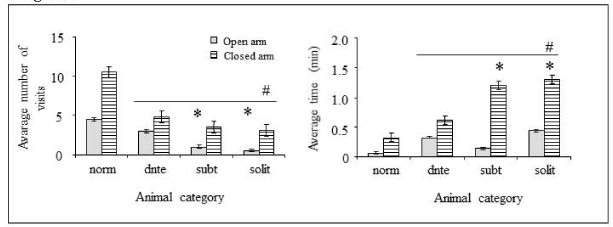
Elevated plus-maze results (Figure 4) correlated with the data obtained by the previous test. Dominant and subordinate mice showed decreased activity as measured by the number of entries and presence time in the open or closed arms when compared to normal mice (Figure 4A,B). Furthermore, subordinate animals demonstrated a high decreased number of entries into the open arms when compared with the normal and the dominant mice (Figure 4A). Concerning exploratory activity, the time remaining in closed arms was higher for the subordinate mice. These values, in number of visits or time in arms, were similar for subordinate and solitary mice (Figure 4B).
Discussion
Our results, in agreement with data from literature, indicate that groups of male mice in adulthood may present PAB [7]. The aggressive behavior can have various causes. The genetic influence may be related to segments of chromosomes with effects on aggressive behavior. This approach is illustrated by the effect of the male-specific part of the mouse Y chromosome on aggressive behavior. It´s proposed that a positional candidate for this effect is Sry [22]. Testosterone also increases adrenal corticoid hormone (ADH) levels in the medial amygdala, lateral hypothalamus, and preoptical medial area, which are involved in aggressive behavior [10]. Neurotransmitter expression, as well as excessive aggressive and impulsive traits of neuronal NO synthase knockout (nNOS-/-) mice were shown to be caused by reductions in serotonin (5-HT) turnover and deficient 5-HT1A and 5-HT1B receptor function in brain regions regulating emotion [23]. We believe that the presence of aggressive behavior and social dominance in adult male mice may be the result of these factors together.
However, one question still needs to be clarified. Why would the PAB appear in some groups and not others, under the same environmental conditions? Issues such as sexual competition [24] and the inclusion of intruders [25] was described in the literature, but this did not happen in our study. Several studies have described social dominance in male mice and related the description of dominant and submissive categories [26,27].
The motor and exploration activity of the mice is born of the requirement for information related to the new environment. The animal acquires information in two ways, evaluated by testing in the open field and the elevated plus-maze. Horizontal displacement, measured by the number of spaces crossed (motor activity) or displacement in arms (open and closed) in the elevated plus-maze, respectively. Exploratory activity was measured by the number of vertical displacements (rearing) while the animal is anywhere in the arena and time spent in the arms. The results for the two tests were similar. Our results showed that dominant and subordinated mice showed decreased motor and exploratory activity in relation to animals without the PBA, that is, despite presenting different categories, the stress promoted by aggressiveness affects individuals of both categories. So, decrease in activity may be related to the chronic stress caused by the aggressive behavior. Furthermore, subordinate animals showed serious decreased in the activity compared with the dominant mice and similar animals in social isolation, what can we characterize as a state of depression (depression-like) in booth categories [28].
Individual housing has also frequently been reported to be uncomfortable for mice and has even been used as a model for social deprivation in man [29]. It may cause both physiological and behavioral abnormalities, referred to as ‘isolation syndrome’ [30]. The clinical depression, understanding of the etiology behind these disorders is far from complete, though theories involving various neurotransmitter systems have been advocated [31]. The symptoms - reduced activity of the submissive and isolated animals - suggest a connection to neurotransmitter imbalance similar to the clinical signs of depression in humans [32]. In consequence, these animals show reduced motor and exploratory activity.
Conclusion
We still do not know the cause of the appearance of PAB in some groups of male mice. The high and/or low level of (mainly motor) activity of the animals comes after the process of social dominance or as consequence of this. Are there two types of social dominance that could arise from the activity level of individual mice? Our goal is to propose a model of maintenance of male mice in the laboratory based on their motor and exploratory activity designed to minimize aggressive behavior. We can conclude that animals which have aggressive disorders in their group show alterations in their motor and exploratory activity and similar with isolation social behavior in suggest depression-like condition.
- Russel W and Burch R. (1992) The principles of humane experimental technique. England: Universities Federation for Animal Welfare, 238.
- Nevalainen T, Kemppinen N, Meller A (2006) Refinement alternative for animal housing - enrichment. ALTEX 23: 93-95.
- Baker K (2007) Enrichment and primate centers: closing the gap between research and practice. J Appl Anim Welf Sci 10: 49-54. Link: http://bit.ly/2MKeAeS
- Olsson IA, Dahlborn K (2002) Improving housing conditions for laboratory mice: a review of "environmental enrichment".Lab Anim 36: 243-270. Link: http://bit.ly/2Zyt45n
- Gonder JC, Laber K (2007) A renewed look at laboratory rodent housing and management. ILAR J 48: 29-36. Link: http://bit.ly/33gJHoz
- Oliveira G, Santana R, Batista W, Soeiro M (2007) Bem estar de camundongos utilizados em laboratório de pesquisa – avaliação do comportamento individual e social. Revista Universidade Rural - Série Ciência da Vida 145.
- Van Loo PL, Van Zutphen LF, Baumans B (2003) Male management: Coping with aggression problems in male laboratory mice. Lab Anim 37: 300-313. Link: http://bit.ly/2YL9r97
- Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, et al. (2006) Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci 26: 2335-2342. Link: http://bit.ly/2Yq2E9d
- Kudryavtseva NN, Amstislavskaya TG, Kucheryavy S (2004) Effects of repeated aggressive encounters on approach to a female and plasma testosterone in male mice. Horm Behav 45: 103-107. Link: http://bit.ly/2GPNVKa
- Giammanco M, Tabacchi G, Giammanco S, Di majo D, La Guardia M (2005) Testosterone and aggressiveness. Med Sci Monit 11: RA136-45. Link: http://bit.ly/2T5YqxO
- Guillot PV, Chapouthier G (1996) Intermale aggression and dark/light preference in ten inbred mouse strains. Behav Brain Res 77: 211-213. Link: http://bit.ly/2MyecAi
- Nelson RJ, Trainor BC, Chiavegatto S, Demas GE (2006) Pleiotropic contributions of nitric oxide to aggressive behavior. Neurosci Biobehav Ver 30: 346-355. Link: http://bit.ly/31kA46h
- Dirocco DP, Xia Z (2007) Alpha males win again. Nat Neurosci 10: 938-940. Link: http://bit.ly/2KgZHzj
- Bragin AV, Osadchuk LV, Osadchuk AV (2006) The experimental model of establishment and maintenance of social hierarchy in laboratory mice. Zh Vyssh Nerv Deiat Im I P Pavlova 56: 412-419. Link: http://bit.ly/2YL6wwN
- Lalonde R, Strazielle C (2008) Relations between open-field, elevated plus-maze, and emergence tests as displayed by C57/BL6J and BALB/c mice. J Neurosci Methods 171: 48-52. Link: http://bit.ly/2OPwcZs
- Liu GX, Cai GQ, Cai YQ, Sheng ZJ, Jiang J, et al. (2007) Reduced anxiety and depression-like behaviors in mice lacking GABA transporter subtype1. Neuropsychopharmacology 32: 1531-1539. Link: http://bit.ly/2T8iRds
- Binder E, Droste SK, Ohl F, Reul JM (2004) Regular voluntary exercise reduces anxietyrelated behaviour and impulsiveness in mice. Behav Brain Res 155 : 197-206. Link: http://bit.ly/2GNfHad
- Kalueff AV, Jensen CL, Murphy DL (2007) Locomotory patterns, spatiotemporal organization of exploration and spatial memory in serotonin transporter knockout mice. Brain Res 12: 87-97. Link: http://bit.ly/2YD5eYY
- Palanza P (2001) Animal models of anxiety and depression: how are females different? Neurosci Biobehav Rev 25: 219-233. Link: http://bit.ly/33ft7oL
- Broadhurst PL (1960) Experiments in psychogenetics. In: EISENK, HJ Experiments in Personality. London: Routledge and Kegan Paul 31-71.
- Pellow S, Chopin P, File SE, Briley M (1985) Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14: 149-167. Link: http://bit.ly/33b7Qwx
- Maxson SC (1996) Searching for candidate genes with effects on an agonistic behavior, offense, in mice. Behav Genet 26: 471-476. Link: http://bit.ly/2OFxxC4
- Chiavegatto S, Nelson RJ (2003) Interaction of nitric oxide and serotonin in aggressive behavior. Horm Behav 44: 233-241. Link: http://bit.ly/2M1DW8L
- Simpson ER, Davis SR (2000) Another role highlighted for estrogens in the male: sexual behavior. Proc Natl Acad Sci U S A; 19: 14038-14040. Link: http://bit.ly/2YrDt6a
- Nakamura K, Kikusui T, Takeuchi Y, Mori Y (2007) The critical role of familiar urine odor in diminishing territorial aggression toward a castrated intruder in mice. Physiol Behav 90: 12-517. Link: http://bit.ly/2M40ghN
- Avitsur R, Kinsey SG, Bidor K, Bailey MT, Padgett DA, et al. (2007) Subordinate social status modulates the vulnerability to the immunological effects of social stress. Psychoneuroendocrinology 32: 1097-1105. Link: http://bit.ly/2T79CKt
- Dubrovina NI (2006) Effects of activation of D1 dopamine receptors on extinction of a conditioned passive avoidance reflex and amnesia in aggressive and submissive mice. Neurosci Behav Physiol 36: 679-684. Link: http://bit.ly/2KiNr1j
- Bartolomucci A, Palanza P, Sacerdote P, Panerai AE, Sgoifo A, et al. (2005) Social factors and individual vulnerability to chronic stress exposure. Neurosci Biobehav Rev 29: 67-81. Link: http://bit.ly/2YITeRs
- Brain P (1975) What does individual housing mean to a mouse? Life Sciences 16: 187- 200. Link: http://bit.ly/2YJokZs
- Hol T, Van den Berg CL, Van Ree JM, Spruijt BM (1999) Isolation during the play period in infancy decreases adult social interactions in the rat. Behavioural Brain Research 100: 91-97 Link: http://bit.ly/2YEzP4o
- Malatynska E, Rapp R, Harrawood D, Tunnicliff G (2005) Submissive behavior in mice as a test for antidepressant drug activity. Pharmacol Biochem Behav 82 : 306-313. Link: http://bit.ly/2MCHD45
- Tunnicliff G, Malatynska E (2003) Central GABAergic systems and depressive illness. Neurochem Res 28: 965-976. Link: http://bit.ly/2YH4ttR
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
