Ann Cytol Pathol
Cancer Stem Cells and Nanomedicine
Entela Shkembi1*, Nicola Daniele2, Francesco Zinno2 and Gallo Emiliano Omar3
2Department of Molecular Biology and Biotechnology CryoLab Srl, University of Rome Tor Vergata, Italy
3Gynecology and Obstetrics, University Hospital Sassari, Italy
Cite this as
Shkembi E, Daniele N, Zinno F, Omar GE (2016) Cancer Stem Cells and Nanomedicine. Ann Cytol Pathol 1(1): 048-053. DOI: 10.17352/acp.000007Introduction
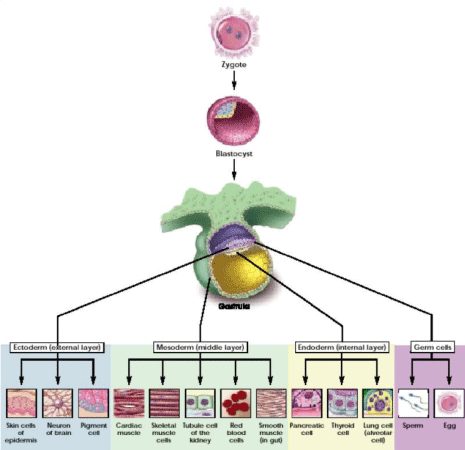
In humans, as in all forms of multicellular life, tissue regeneration is a physiological process of cell renewal necessary for the survival of the organism. For tissues such as blood and skin is a daily replacement with younger cells that replace the old ones. In other tissues, such as brain and heart, this replacement is much more reduced. At the base of these regenerative processes there are populations of reserve cells located in different tissues, the more abundant the greater the regenerative capacity and the necessity of that tissue. Immature cells capable of self-maintained, that is, endlessly multiplied by generating identical cells to themselves. While replicating, stem cells also retain the ability to specialize in different mature cell types of the tissues and organs in which they are located. It is through for the double activity that stem cells of our body replicate and specialize every day to replace worn cells and allow the body to survive. It was the Russian scientist Alexander Maximov in 1906 to introduce for the first time the term ‘stammzelle’ (from “Stamm”, jamb, and “zelle”, cell) to refer to individual cells of a cell lineage progenitors. And it is of the fifties the first experimental evidence of the existence of stem in the body. However, the searchlights have been turned on these cells only since 1998, when a special stem cell has been isolated from human blastocyst (an early stage of embryo development): the embryonic stem cell.
Today, both the stem of our adult tissues both embryonic stem cells of the blastocyst (Figure 1), represent a powerful tool with which to understand how they form and get sick our bodies, nurturing the hope, in some cases already become reality, which in the future may be employed in transplantation strategies to replace cells lost due to injury or illness.
The common origin of all blood cellular component and specific and not specify immunity is the hematopoietic stem cell. The mature blood cells are destined to live a few hours to many weeks, before being seized and destroyed. On one day, therefore, billions of cells must be produced to replace those destroyed. The need for a continuous supply of hematopoietic cells derived from the fact that the blood cells must be replaced continuously during the span of life
Recent studies have shown that human stem cells:
• located at a frequency of 1: 104 bone marrow cells;
• able to regenerate itself thanks to a process of “self-renewal”;
• metabolically quiescent and rarely comes into the cell cycle;
• Helps to expand the pool of “multipotent progenitors” capable of developing all the hematopoietic lineages, eventually losing, at some point, their ability to differentiate into lymphoid sense and become “the commissioned progenitors Myeloid-Erythroid”.
So that you have a good hematopoiesis requires a hematopoietic microenvironment suitable. So important are the relationships that are established in the marrow from stem cells and osteoclasts, adipocytes, stromal cells, vascular and extracellular matrix system whose function is not only to anchor hematopoietic precursor cells with the right-integrin interaction integrin (VLA-4 and VCAM-1) but also to compartmentalize the soluble factors, such as GM-CSF, IL-3, T cell growth factor, spatially orienting hematopoietic precursor cells in the erythrocyte and granulocyte islets.
Stem cells: classification and origin
Stem cells are primitive cells, unspecialized, with the unique potential to differentiate into any other cell type in the body. These two basic characteristics that distinguish them from other cells:
• their ability to self-renew, then the ability to make an unlimited number of replicative cycles while maintaining the same differentiation stage;
• The multipotency, the ability to give rise to one or more cell species.
Stem cells are normally quiescent cells (G0 phase block in the cell cycle) or characterized by slow cell cycle [1], preserved in an undifferentiated state until it is requested their participation in the normal physiology of the body. The asymmetric division of stem cells leads to the formation of a stem cell identical to the parent cell, that so maintains the stem cell pool and a progenitor cell partially differentiated and incapable of self-renewal (not stem), but has a large potential of replication.
This strategy, which limits the number of replicative events which a stem cell undergoes, is probably based on two important principles linked together:
• strict control of the number of stem cells: each stem cell occupies its own biological niche defined by a complex network of biochemical signals, which probably provide the stem cells necessary information about the appropriate time to replicate;
• Retention of integrity of the genome of the stem cells: a low number of repetitions reduces the risk of damage to DNA.
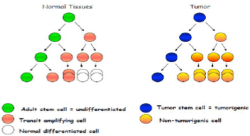
Physiologically, it is very important that it is maintained a constant number of stem cells and, except for the expansion phase that has embryogenesis or for the phase of repair of severe tissue damage, is precisely the asymmetric division of stem cells to allow that to happen (Figure 2).
Mutations of stem cells are extremely dangerous, as they are transmitted to all generations of daughter cells derived from the stem cell. Contrarily, progenitor cell’s mutation affects only one single generation of cells that after a certain time it will be eventually replaced anyway. Moreover, these alterations can induce stem cell to be degenerated in neoplastic sense, thus becoming a tumor stem cell [2], i.e. a type of cell probably responsible for the continuous provision for new cancer cells that characterizes the development and especially the recurrence of the tumors.
Stem cells can also be classified according to origin, as adults or embryonic [3]:
• Adult stem cells are unspecialized cells found between the specialized cells of a specific tissue and are mostly multipotent,called somatic (from Greek “σωμα” Soma = body), because they do not necessarily come from adults, but even from children or umbilical cords;
• Embryonic stem cells are obtained for the culture medium, derived from the internal cells of a blastocyst. Already in 1858, Rudolf Virchow proposed the hypothesis that the originasse cancer from embryonic stem cells. This concept, based on histological similarity of tumors and embryonic tissues, was extended by Cohnheim and during, which suggested that adult tissues contain embryonic residues that normally remain in the dormant form, but which may be activated becoming cancer [4].
While it is now clear the role of stem cells in normal physiology, it is more recent evidence their involvement in the malignant transformation.
Despite the enormous progress made in cancer research, there are still a number of open questions. For example, what causes some cancers are more difficult to eradicate than others? Why are some cancers more resistant to therapy? And why some tumors are aggressive and others are not? To answer these questions the attention has focused on stem cells, which could be the target of malignant transformation as they are characterized by a long service life, and be able to accumulate mutations which then lead to cell transformation and able to “support” the tumorigenicity process having the ability to divide indefinitely.
Cancer stem cells
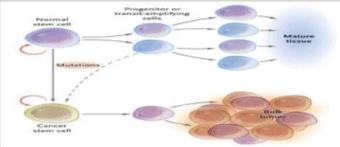
Despite the ongoing debate on the existence of cancer stem cells, there is no denying that most tumors are heterogeneous and show functional and phenotypic differences at the cell population. These differences may result from the evolution followed by clonal differentiation of tumor stem cells or genetic instability [5]. In addition, cancer stem cells may vary between different cancer patients and can constantly change with disease progression. Therefore, for the prevention and treatment of cancer, we need to identify and characterize these subpopulations. Cancer stem cells could ask some properties. Subpopulations of CSC can be isolated according to the profiles of cell surface markers, show a greater resistance to radiotherapy and conventional chemotherapy [6]. These features imply the existence of distinct populations of tumor cells that have a property of self-renewal and the potential to cause tumors with a limited number of cells. Cancer stem cells were first identified in the acute myeloid leukemia (AML). It has been seen that a sub-population of leukemic cells with phenotype CD34 + / CD38- was responsible the onset of leukemia in mice, when inoculated into a mouse NOD / SCID mice (Figure 3). Cancer stem cells have also been found in various solid tumors, as in breast cancer, where stem cells have been described with phenotype CD44 + / CD24- and aldehyde dehydrogenase 1 (ALDH1) + (7). Singh and colleagues have shown that, by inoculating in NOD / SCID 100 cells positive for the stem cell marker CD133, it had the appearance of tumors.CD44 is a ubiquitous protein, recently found overexpressed in various tumor tissues.The expression of CD44 is a marker indicative for effector T cells of memory. The proliferation of memory cells (activation) may be tested in vitro chemically marking them with CFSE, in some tumors and CD44v6 was used as a target for cancer therapy. Subsequently, CSCs have been identified in breast cancer and certain brain tumors. These discoveries have given way to the identification of stem cells in many other solid tumors: in 2007 were discovered stem cells in the intestine tumors, pancreatic, head / neck, followed in subsequent years by the stem of prostate cancer, lung, thyroid, stomach, kidney, ovary and bladder.
Cancer stem cells and the progenitor
Growing evidence suggests the existence of a dynamic, two-way conversion balance between CSC and tumor progenitors. On one side, the CSC could self-renew and generate multiple differentiated tumor progenitor cells hierarchically through the asymmetric replication. Furthermore, the cancer progenitor cells had the ability to differentiate and acquire a stem cell phenotype that risposse to a series of factors, such as:
• the microenvironment;
• transduction pathways;
• the molecular circuits;
• epigenetic modification;
Proia et al. [8], have demonstrated that the destiny of progenitor cells and the tumor phenotype could be affected significantly by the genetic background of populations of patients and incidence rates. Understand the links between the CSC and progenitor cells of the cancer is fundamental for the development of therapeutic strategies for cancer, by the inactivation of endogenous de-differentiation mechanisms. Given these characteristics, it is possible that cancer stem cells derived from the same normal stem cells as a result of a mutation. Other lines of research argue that cancer stem cells may arise from progenitor cells mutated. A tumor progenitors show a low self-renewal capacity and a higher probability of terminal differentiation compared to CSC. Several studies have indicated that most of the leukemic cells it descended from a small pool of progenitor cells to high activity proliferative [9], as occurs in acute myeloid leukemia (AML).
These progenitor cells have two main properties as follows:
• Active cycle and proliferation in vivo while most of the cell cycle daughters outputs cells are unable to proliferate in vitro;
• Differentiation in vitro limited extent, and the ability to be analyzed by means of special surface markers in the different stages of differentiation (Figure 3).
Growing evidence indicates that using targeted therapies, to control the cancer is detected to the CSC and towards cancer progenitor cells show different levels of sensitivity. The stem cells of chronic myeloid leukemia are insensitive to tyrosine kinase inhibitors such as imatinib, dasatinib and nilotinib, while the leukemic progenitor cells [10], are rather sensitive to these drugs. The mechanism to escape the inhibition of imatinib in chronic myeloid leukemia stem cells could be mediated through the activation of survival pathways such as Wnt/ β-catenin and AKT / PTEN [11]. The markers used were the CD133 and CD24, markers of undifferentiated cells, frequently coupled with migratory molecules such as CD44 + / CD24. Growing evidence have shown that the surface markers expressed by CSC and progenitor cells from cancerous cells are different in some way. In 2003, high expression of CD44 / CD24low + / - have been detected in the stem cells of breast cancer. However, it was unclear whether the CD44 and CD24 could distinguish tumor cells from not normal. Subsequently these CSC-like cells occurred intrinsically resistant to conventional chemotherapy and ionizing radiation. A study by Venugopal et al. [12], showed that the initial cells of the brain tumor can generate all types of neural cells through differentiation. It ‘been shown that the antigen of neuronal stem cells, CD133 is expressed in TICs (tumor initiating cells) of cerebral origin derived from pediatric medulloblastoma and from’ astrocytoma. During this time, the stem cells and early progenitor CD133 + cells lose their CD133 expression giving rise to progenitor past and finally differentiated progeny. For this reason activate the mechanisms that protect them from the senescence and stress. These mechanisms include activation of some routes, such as those of BMI-1 and an increased ability to repair DNA damage.
The Pathway involved in self-renewal and oncogenesis
Cancer stem cells produce tumors through self-renewal and differentiation regulated by different routes of transduction. The comprehension of self-renewal mechanisms in CSC is of great importance for the discovery and development of new drugs. The transduction pathways such as Wnt, Sonic-Hedgehog and Notch, which regulate the proliferation and survival of noncancerous neural precursors, are often activated in an aberrant in tumors.
The Notch pathway: The Notch pathway transduction is a present cell signaling system in the majority of multicellular organisms, that
• The Notch effect can be observed in the embryonic stem cells of both mice and human, and can be re-created without recourse to genetic engineering: the presence of the Notch protein is sufficient to activate the signals in the cells on which to grow the stem cells.
The activation of this pathway is associated with an increase in the stem cell pool. Begins with the binding of the ligand (Delta-like 1, 3 or 4 or Jagged 1 and 2) with the receptor (Notch 1-4), followed by a proteolytic cleavage of the receptor from the complex of gamma secretase. Inhibitors of this pathway slows the growth of tumors by employees of Notch activation, such as medulloblastoma and T-cell leukemia.
In the embryonic brain tumors inhibition of Notch leads to a selective depletion of tumor stem fraction. In 2010, it was observed that the shape-activated Notch1 and its downstream targets are expressed in cell-OCT4 and SOX2-positive human nasopharyngeal carcinoma [13], suggesting that Notch1 signal is activated in these cells in order to give place at the molecular regulation of cancer stem / progenitor cells in nasopharyngeal carcinoma.
The Wnt/β-Catenin Pathway
Its activation expands the stem cell pool, while its suppression inhibits proliferation of stem cells.
The Wnt/β-Catenin Pathway send molecular signals in the pathway, and the function of βcatenin is critical in this street. The Wnt/β-Catenin Pathway, has been demonstrated to be important in regulating the self-renewal of hematopoietic stem cells [14] and genes involved in cell cycle regulation: all characteristics that define a stem cell [15]. E ‘was also recently proposed that TSC be part of the WNT pathway, to bind to the degradation complex of β-catenin, and act negatively regulating the transcription of WNT target genes; phosphorylation of Akt / PKB and / or Erk-1/2 would prevent the formation of the TSC complex and this would result in an increase in the transcription of these genes involved in Cycle adjustment as c-myc, n-myc or cyclin D1 [16]. In the absence of Wnt signaling, β-catenin is rapidly degraded by multiprotein complex APC / Axina / GSK-3β (Glycogen Synthase Kinase-3β).This complex facilitates the phosphorylation of β-catenin by GSK-3β, thus increasing the amount of β-catenin bound. Once β-catenin phosphorylation is linked to the F-box protein β-transducin repeat containing protein, a component of the E3 ubiquitin ligase complex, which makes the poly ubiquitinated and then degradable by proteasome.
The hedgehog Pathway
The Sonic Hedgehog (SHH) is one of three proteins of the hedgehog family; the other two are the Desert Hedgehog (DHH) and Indian Hedgehog (IHH). SHH is one of the most studied ligands of dell’hedgehog signaling pathways (Hedgehog signaling pathway); this pathway is critical in the control of organogenesis in vertebrates, for example, in the differentiation of fingers and limbs, in the organization of the nervous system and teeth. Sonic Hedgehog is the best sample morphogenetic molecule: the molecule diffuses producing a concentration gradient, the embryo cells develop into different tissues, according to the local concentration of SHH. This molecule remains important even in adulthood. Controls cell division of adult stem cells and is involved in some types of cancer.
Medical “nanomedicine” for the therapy to the cancer stem cells
Nanomedicine is the medical application of the possibilities deriving from nanotechnology. It then deals with all the knowledge and technologies that have a medical use in the order of nanometers in size (1-100 nm). Working at this scale nanotechnology alters the traditional distinction between biology, chemistry. Currently, nanotechnology applied to most promising medicine and already being tested concern: the development of nano-carriers for targeted drug delivery and the “lab-on-a-chip” and other types Micro Electro Mechanical Systems (MEMS), usable for diagnostic purposes. The nanocarriers are one of the best alternatives for administration of medicines chronic disease care, who need continuous treatments, usually at high doses, which often involve significant side effects. In this regard, it should be emphasized that there is great interest in developing new systems of “nanodelivery” for drugs that are already on the market, especially anticancer drugs. Among the possible values to be used for drug delivery are those made with gold nanoparticles, silica, or iron oxides, conjugated with dendrimers or with peptide and / or antibodies that facilitate the recognition of the molecular target to which head. These systems show great potential for use both diagnostic and therapeutic. These carriers function as a principle similar to that missile designed by NASA to reach the moon: the particles dissociate sequentially during their travel through the human body, overcoming so all biological barriers in the body, including the immune system, the vessel walls, and adjacent to the tumor tissue. Today, we got to have bio MEMS called by different terms (DNA chip, biochips, micro-plants, microarray, bioMEMS, cell-chip), representing microscopic “laboratories” interactive remote control, able to collect and transmit data in the patient’s body. The nano-vehicles extend movement and improve the bio-distribution of the incorporated drug, resulting in a better accumulation in tumors through a process known as higher permeability and retention. The virtue of the nano-vehicles is that they can be adjusted using molecules without loss of activity. Further, these are used to encapsulate chemotherapeutic, complexing unfavorable domains all’interzione with the drug compared and the body. As a result, the targeted nanomedicine to CSC requires that there be a multidisciplinary cooperation to develop new agents and an accurate data interpretation obtained from many different disciplines. An efficient route for the administration of the drugs, using nano-vehicles loaded with medication, is the penetration through the cell membrane, in particular in the 1chemoresistant tumor cells.
Development of nanomedicine for CSC
To enhance the therapeutic effect on the CSC, in the nano-scale drugs they have enabled the development of many new strategies to overcome the shortcomings of many well-known anticancer drugs, such as:
• extrusion of the drug,
• low solubility in water
• instability
• high nonspecific toxicity [17,18]
These nano-particles include polymeric and non-polymeric micelle systems. The polymeric micelles with the core-scell structure are formed by the self-assembly of amphiphilic grafts in water, providing a significant advantage for the delivery of cytotoxic agents for cancer [19]. Previous studies have shown that the cellular uptake of drugs is increased by using nano-vehicles compared to the free drug. For example, estato developed a new micellar formulation of oxaliplatin encapsulated in a vesicle of chitosan (CSO-SA / OXA micelles). The absorption of nano-vehicles can be via endocytosis in which the free drug is internalized in cancer cells for molecular diffusion. An efficient route for the administration of the drugs, using nano-vehicles loaded with medication, is the penetration through the cell membrane, in particular in the chemo resistant tumor cells. Zhou et al have described that the resistance of CML cells CD34 + and CD34 + CD38- can be overcome by using synthetic low-density lipoprotein particles (SLDL) [20]. The SLDL are prepared using a solvent evaporation method on a mixture consisting of phosphatidylcholine, triolein, cholesterol, and cholesteryloleate in a molar ratio of 3: 2: 1: 1, synthetic lipophilic rispettivamente. Lipophilic synthetic peptides specific for the receptors of low-density lipoproteins have been used to hit the CML cells. For example, curcumin has been described as effective towards the CSCs in vitro colon cancer. However, its application in the treatment of cancer is limited by the high hydrophobicity, instability, and gives a current pharmacokinetics. Drugs of nano-metric scale offer an innovative approach to overcome these problems.
Conclusions and Future Prospects
During those few seconds we use to say the word ‘nanotechnologies’ our hair grew in ten nanometers. We are talking about around the billionth of a meter in size, these nano-particles that now are found everywhere, in cosmetics, in the chips, and nano-robots that are becoming the great promise of future medicine.Nanotechnology allows us to use less drugs, to release them in the right place, ie in the cells to be treated, thereby reducing the side effects of therapy, and to cure diseases like certain cancers today uncontrollable. They are hi-tech nanoparticles, capable to overcome the defensive barriers of cancer so far impervious to traditional chemotherapy. They are able to cross the dense mass surrounding the tumor and transfer the medicinal product selectively in diseased cells, at higher concentrations, and without damaging the healthy tissues, one of these nanomedicines, paclitaxel, and ‘have already’ successfully used in breast cancer.
Research and development aimed at the discovery of new products for the treatment of cancer, the search for synergies with the biopharmaceutical sector companies and alliances involved in the oncology sector and the scientific community, the area growth of research services for the development preclinical and pharmaceutical, discovery and implement innovative cancer therapies, not only thanks to the exploitation of the technological platforms and internal expertise, but also by creating partnerships with the pharmaceutical industry. Mauro Ferrari is considered the father of nanomedicine, for joint research with the National Institute of Tumors. In particular, the heterogeneity of the tumor cell performance can help define tumor resistance to conventional therapies. Nevertheless, the design of therapies nanomedicine versus the CSC has proved complex, perhaps because CSCs in the same type of cancer are phenotypically and functionally heterogeneous, partly because of the nonspecific nature of CSC markers used for targeting.That same year, the research team have discovered a molecule called the Ttk / Mps1, a target that regulates mitosis and is overexpressed in many tumors and is considered a new and very promising target in oncology. Profile genomic differences have recently been exploited to personalize medicine and CSC, can facilitate the nano-medicine indiuviduale specific and dose selection for improved effectiveness of cancer treatment and patient prognosis.
- Alvero AB1, Chen R, Fu HH, Montagna M, Schwartz PE, et al. (2009) Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Cell Cycle 8: 158–166.
- Fang JS1, Deng YW, Li MC, Chen FH, Wang YJ, et al. (2007) [Isolation and identification of brain tumor stem cells from human brain neuroepithelial tumors]. Zhonghua Yi Xue Za Zhi 87: 298–303.
- Baker DE, Harrison NJ, Maltby E, Smith K, Moore HD, et al. (2007) Adaptation to culture of human embryonic stem cells and oncogenesis in vivo. Nat Biotechnol 25: 207–215.
- Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414: 105–111.
- Dalerba P, Cho RW, Clarke MF (2007) Cancer stem cells: models and concepts. Annu Rev Med 58: 267–284.
- Li X1, Lewis MT, Huang J, Gutierrez C, Osborne CK, et al. (2008) Intrinsic resistance of tumorigenic breast cancercells to chemotherapy. J Natl Cancer Inst 100: 672–679.
- Yeung TM, Gandhi SC, Wilding JL, Muschel R, Bodmer WF (2010) Cancer stem cells from colorectal cancer-derived cell lines. Proc Natl Acad Sci U S A 107: 3722–3727.
- Proia TA, Keller PJ, Gupta PB, Klebba I, Jones AD, et al. (2011) Genetic predisposition directs breast cancer phenotype by dictating progenitor cell fate. Cell Stem Cell 8: 149–163.
- Löwenberg B1, Touw IP (1993) Hematopoietic growth factors and their receptors in acute leukemia. Blood 81: 281–292.
- Griffin JD, Lowenberg B (1986) Clonogenic cells in acute myeloblastic leukemia. Blood 68: 1185–1195.
- Palomero T, Dominguez M, Ferrando AA (2008) The role of the PTEN/AKT Pathway in NOTCH1-induced leukemia. Cell Cycle 7: 965–970.
- Venugopal C, Li N, Wang X, Manoranjan B, Hawkins C, et al. (2012) Bmi1 marks intermediate precursors during differentiation of human brain tumor initiating cells. Stem Cell Res 8: 141–153.
- Wang J, Guo LP, Chen LZ, Zeng YX, Lu SH (2007) Identification of cancer stem cell-like side population cells in human nasopharyngeal carcinoma cell line. Cancer Res 67: 3716–3724.
- Kim M, Turnquist H, Jackson J, Sgagias M, Yan Y, et al. (2002) The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin Cancer Res 8: 22–28.
- Huang EH, Hynes MJ, Zhang T, Ginestier C, Dontu G, et al. (2009) Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res 69: 3382–3389.
- Tetsu O, McCormick F (1999) Beta-catenin regulates expression of cyclin d1 in colon carcinoma cells. Nature 398: 422–426.
- Chen B, Sun Q, Wang X, Gao F, Dai Y, et al. (2008) Reversal in multidrug resistance by magnetic nanoparticle of Fe3O4 loaded with adriamycin and tetrandrine in K562/A02 leukemic cells. Int J Nanomedicine 3: 277–286.
- Liu C, Zhao G, Liu J, Ma N, Chivukula P, et al. (2009) Novel biodegradable lipid nano complex for siRNA delivery significantly improving the chemosensitivity of human colon cancer stem cells to paclitaxel. J Control Release 140: 277–283.
- Eyler CE, Rich JN (2008) Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J Clin Oncol 26: 2839–2845.
- Zhou P, Hatziieremia S, Elliott MA, Scobie L, Crossan C, et al. (2010) Uptake of synthetic Low Density Lipoprotein by leukemic stem cells – a potential stem cell targeted drug delivery strategy. J Control Release 148: 380–387.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
