Archives of Clinical Nephrology
Acute kidney infarction: Not so rare renal disease. A single-center experience with endovascular fibrinolytic therapy
Scarpioni R1*, De Amicis S1, Bodini FC2, Albertazzi V1 and Michieletti E2
2Department of Radiology, “Guglielmo da Saliceto” AUSL Hospital, Via Taverna 49, 29121 Piacenza, Italy
Cite this as
Scarpioni R, De Amicis S, Bodini FC, Albertazzi V, Michieletti E (2022) Acute kidney infarction: Not so rare renal disease. A single-center experience with endovascular fibrinolytic therapy. Arch Clin Nephrol 8(1): 001-006. DOI: 10.17352/acn.000058Copyright License
© 2022 Scarpioni R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Renal Infarction (RI), a rare cause of renal damage characterized by the abrupt interruption of flow in the renal artery, is often recognized late or may even remain undiagnosed since symptoms are non-specific and may be confused with other pathologies, such as pyelonephritis or nephrolithiasis. In situ thrombosis and thromboembolism are the main causes, but often the real cause is, gf unrecognized. The disease is often underdiagnosed and the diagnosis of certainty can be established with ultrasonography Doppler of renal arteries or with second-level diagnostic tools (contrast-enhanced computer tomography, magnetic resonance with gadolinium, and renal scintigraphy) or third level tests (renal arteriography). The therapeutic approach depends on the cause of RI, from the time from onset of ischemia, from the presence of kidney function impairment, and may include systemic anticoagulant therapy, renal angioplasty with or without stenting, loco-regional endovascular fibrinolytic therapy or surgery, as the last chance. In literature, there are neither guidelines nor evidence about any treatment superiority. Here we describe a paradigmatic case in a 51-years-old man hospitalized because of sudden flank pain: the clinical picture, the high serum level. Moreover, we report our 7-years’ experience with 24 cases of RI, mean age 70 /±15 years, 14/24 men, 16/24 presented with hematuria, frequently associated with the history of CKD (16/24). Fifteen of them (62%) were classified as idiopathic and 9 of them were successfully treated with endovascular fibrinolytic treatment. A review of the literature is also provided.
Introduction
Renal infarction (RI) is a rare cause of renal damage, characterized by the abrupt interruption of flow in the renal artery or in one of its major branches. The etiology of renal infarction depends on several causes, alongside the embolic forms associated with atrial fibrillation or endocarditis, states of hypercoagulability inducing thrombosis in situ, spontaneous renal artery dissection, or trauma: however, often an obvious cause is not recognized.
RI is often recognized late or it may even remain undiagnosed since symptoms are non-specific and may resemble other, more frequent, pathological conditions, such as pyelonephritis and renal colic secondary to nephrolithiasis [1]. The most frequent symptoms at onset are usually flank or abdominal pain, often so intense that opioid therapy may be required, fever, hematuria, and the onset of nausea or vomiting, but they depend on the time since onset of ischemia, the longer the time to onset, the lower the extent of the symptoms. Hypertension is frequently present [2]. Specific clinical situations and laboratory blood tests may help in the choice of diagnostic procedures if there are predisposing conditions such as cardio-embolic diseases (atrial fibrillation, cardiovascular disease, heart valve disease), pro-thrombotic conditions, traumas, or other comorbidities that lead to arterial vessels damage [3]. Diagnosis of certainty can be established with ultrasonography and doppler exams targeted on renal arteries, for which the operator’s experience is required, but often a second-level examination is required (contrast-enhanced computer tomography-CT, magnetic resonance with gadolinium, renal radioisotope scan to identify a decrease in renal perfusion) or third level tests (arteriography) [4-6].
The therapeutic approach depends on the cause of RI and includes systemic anticoagulant therapy, percutaneous endovascular therapy with or without stenting, and/or loco-regional endovascular fibrinolytic therapy, or, finally, surgery, as the last chance. There are no guidelines or comparing studies guiding toward the best therapeutic behavior . Literature reports only sporadic cases or case series on this topic. It is true, though, that systemic blood declotting is mandatory in most cases while the surgical approach is never the first choice, except in conditions requiring intervention for other clinical reasons, such as trauma [7].
Here a paradigmatic clinical case of acute idiopathic RI without kidney impairment in a young man, will be described and also reported our experience on 24 patients with RI in lasting 7 years with therapeutic approaches.
Clinical case
A 51-year-old man, employed as a worker, entered the emergency room because of a severe pain in his right flank irradiated in the ipsilateral iliac fossa, that had arisen 12 hours before. He denied urinary disorders nor alterations in the urine, disclaiming recently pharyngitis-tonsillitis episodes. The patient was overweight (body mass index-BMI=30), active smoker (more than 20 cigarettes a day), without any significant medical history except a splenectomy in the past; he did not take medications chronically, notably Non-Steroidal Anti Inflammatory Drugs, NSAIDs. He had no familiar medical history of kidney, cardiac or thromboembolic diseases. He reported having had a fever (not present at the entrance) and pain in his right flank for a few days, so, under medical prescription, he had started a steroid therapy and took 2-3 tablets of ibuprofen. His blood pressure values were 130/70 mmHg, the abdomen was painful on the right side with costovertebral angle tenderness (CVAT) test slightly positive. The electrocardiogram showed sinus rhythm without ischemic signs.
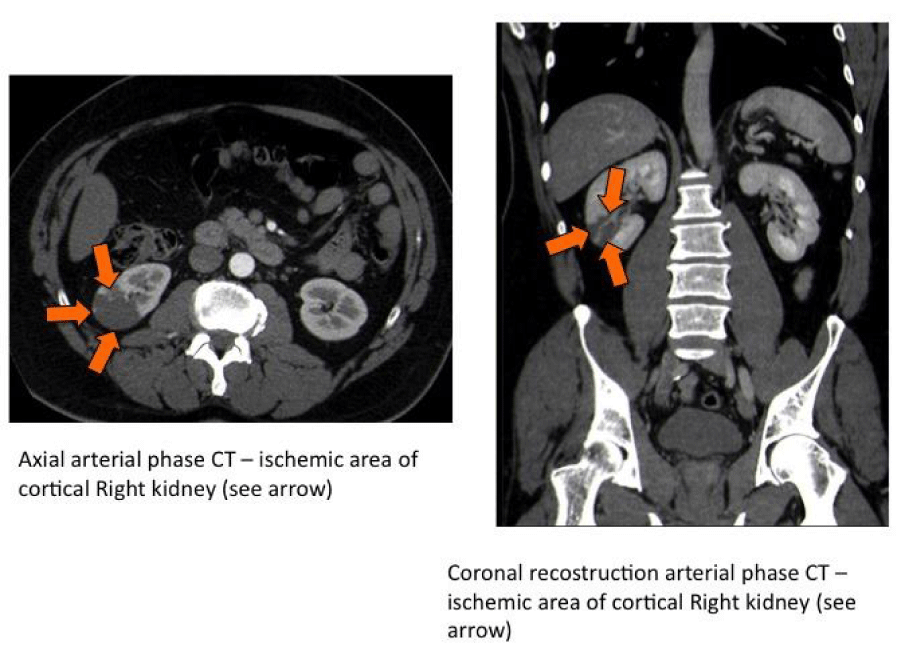
Laboratory blood tests at admission showed neutrophilic leukocytosis (GB 21450/uL, neutrophils 76.6%) with normal renal function (serum creatinine= 0.99 mg/dL, urea= 55 mg/dL, estimated glomerular filtration rate,-eGFR by CKD-EPI formula= 87ml/min); in the limits were hemoglobin, hepatic cytolysis indexes, glycemia , C-reactive protein, platelets, and coagulation tests, while serum LDH, was normal at baseline, while it increased after 12 hours (629U/L, normal values up to 248U/L), usually not associated with nephrolithiasis or pyelonephritis. Urinalysis showed hematuria 2+ and leukocyturia 2+ with moderate albuminuria (10mg/dL). Culture tests on blood and urine were negatives and renal ultrasonography did not show calculi, hydronephrosis, or perirenal collections: the abdominal contrast-enhanced CT showed two hypodense areas (in the middle third and in the lower pole area of the right kidney) of about 3 and 4 cm of maximum length. They were wedge-shaped and with clear margins (Figure 1).
Broad-spectrum antibiotic therapy was started and NSAID and paracetamol were administrated. The diagnosis was of right renal infarction. Concurrently, selective arterial catheterization of the intermediate-posterior branch of the right renal artery was then performed using a coaxial microcatheter together with a local infusion of urokinase (50,000 units) followed by sodium heparin (5,000 units), after that systemically continued and followed in the subsequent days by vitamin K inhibitor. The painful symptoms attenuated a few hours after until completely disappeared after two days: no signs of arrhythmias by electrocardiographic monitoring were found in the days after and blood pressure remained normal. Negative resulted laboratory tests for predisposition to autoimmune and cardiovascular diseases or thrombophilia screening. CT images did not detect atheromatous plaques within the main vascular tree. On the following day’s kidney function indexes remained normal, urinary abnormalities resolved and serum L-lactate dehydrogenase (LDH) levels gradually normalized. However, five days after the angiography procedure a reactivation of the pain in the contralateral left side was observed: in the suspicion of contralateral recurrence of RI, contrast-enhanced CT showed a newly detected hypodense area in the spleen without ischemic lesions at left kidney, while in the right kidney ischemic lesions showed less defined margins and inhomogeneous density with areas of renal parenchyma already revascularized. Anticoagulant therapy and infusion of heparin were continued for a few days with consequent regression of pain. Renal scintigraphy, performed one week later, revealed only a slight reduction in the filtration contribution of the right ischemic kidney (right= 44%, left= 56%) with renal function tests within the normal limits. Six years after the episode the patient is still asymptomatic, he has no recurrence of RI and renal function is within limits without any therapy except oral anticoagulation, as no specific cause has emerged. He definitely stopped cigarette smoking and slightly reduced his body weight.
Results
As a matter of fact, in our relatively small size, single-Center experience in a tertiary-Hospital 24 cases of RI was diagnosed during the last 7 years, a not so small number considering an influx of 300,000 inhabitants in our district. In our hospital from 1 January 2013 to 31 December 2019, 24 diagnoses of renal infarction were done: the diagnosis was collected from the hospital classification system (International Classification of Diseases, 9th revision) of the National hospital admissions database (schema dimissioni ospedaliere-SDO). All medical records with diagnostic tools, therapy, and clinical data were retrieved and analyzed by DAS, AV, and SR. All the patients gave their written informed consent and the local ethics committee of Piacenza Hospital approved the retrospective study. Moreover, according to our hospital policy, all the clinical information can be used for review and research purposes provided that anonymity is warranted Table 1.
In our experience (Table 2 Baseline characteristics of RI patients): the mean age at diagnosis was 70±19 years (range 36-88), 10 were females and 14 males, the diagnosis was done above all by CT (only in 5 patients MRI was used, because of renal impairment) and contrast graphic ultrasound requires an experienced operator and usually does not allow to make a clear diagnosis of certainty and must refer to the CT, a second-level examination. Fifteen among 24 patients were ascribed to idiopathic RI without any apparent cause, and 9 patients underwent interventional endovascular radiologist procedures. Their mean age was 71.2 years, 4 males/5 females. No serious collateral side effects, namely major hemorrhagic episodes were reported.
In our experience endovascular curative procedures were associated with reduction of the kidney ischemic area, determined by enhanced CT, without significant impairment of renal function.
No trauma or renal acute injury was reported. Six patients had a history of cardiac arrhythmia, confirmed by EKG at the entrance. In our experience, severe renal impairment necessitating hemodialysis occurred in eight patients at initial presentation, and five of them definitively required chronic intermittent hemodialysis.
Discussion
The RI is caused by a sudden interruption of blood flow in the renal artery or one of its branches. The main causes can be merged into four groups: see Table 3.
The incidence of RI in the literature ranges between 0.004 and 0.007% among emergency departments diagnosis [2,9] but this data is probably underestimated as symptoms are non-specific and often the diagnosis is hypothesized a posteriori, as per an autopsy study carried out in the far 1940, where the prevalence was 1.4% [10]. In patients with Atrial Fibrillation (AF), RI is more common: in the general Danish population, patients with AF had an increased relative risk of thromboembolic events (4 and 5.7, respectively, among 30,000 patients with AF) [11] while in the previously reported study among 438 patients with cardioembolic renal infarction, almost the half had atrial fibrillation [3].
Systemic fibrinolytic therapy in our experience may be safely used, although this approach is reported to be effective only in a few cases [12], nevertheless the risks of significant bleeding are higher with systemic thrombolytic therapy than with local thrombolysis. The idiopathic forms of RIs not associated with any obvious cause are a considerable proportion, reported to be almost 30% by the above-mentioned manuscript [3] and even up to 60% in another small experience on a small number of 27 patients [13]: in our experience, 16 patients (66% of total) were ascribed to have had idiopathic form, and incidence in line with what is reported in the literature. The association with exposure to cigarette smoking is correlated to these forms of RI where other cardioembolic causes do not emerge [8]. Also in our case, cigarette smoking (known to alter the mechanisms of coagulation by influencing the function of endothelial cells, platelet aggregation, fibrinogen and coagulation factors [14,15] and overweight, were risk factors present in the medical history of our patient. It is noteworthy that acute RI may occur in previously apparently healthy individuals, but also patients with renal pain but without nephrolithiasis: the diagnosis of acute RI should be suspected and enhanced CT should be considered, especially if serum lactate dehydrogenase is elevated because LDH enzyme is too large to be filtered and therefore its excretion rate is normal in not-renal disorders, while in RI there is an increase in the excretion rate from the kidneys.
It is important to emphasize that early diagnosis is associated with a better prognosis [16].
Among causes of risk of thromboembolism Trousseau’s syndrome (cancer-associated thrombosis) is a possible cause, mainly venous rather than arterial, leading to death in cancer patients. The risk of venous thromboembolism is 4- to 7-fold higher in patients with cancer than in those without cancer depending on impaired coagulation, associated with particular cancer or treatment. In our patient clinical examination with the absence of lymphadenomegaly, the negativity of chest radiography, CT, and abdominal ultrasonography, together with the negativity of the main laboratory markers of neoplastic diseases, permitted us to exclude cancer as a potential cause of RI. Moreover, the presence of patent foramen ovale (PFO), a condition due to the small flaplike opening between the right and left upper chambers of the heart (atria), is rarely reported in the literature to be associated with paradoxical embolism through a PFO, leading to renal infarction [17,18]. The diagnosis of right-to-left congenital cardiac shunts can be difficult and cardiac catheterization and angiocardiography are the traditional gold standard for diagnosis, but they are invasive. Also, nuclear scintigraphy using 99mTc-Macro Aggregated Albumin (MAA) may be helpful. In practical clinical often the first-line exam is color flow Doppler echocardiogram, which can detect the flow of blood between the right atrium and left atrium and, as a second-line exam, a transesophageal echocardiogram get a closer look and blood flow through the heart. In our experience, a color flow Doppler echocardiogram was done in all patients and our cardiologists, on the basis of the clinic and the results of the color flow Doppler echocardiogram, did not consider necessary to perform a transesophageal echocardiogram in all patients. Finally, other less common etiologies associated with renal artery injury leading to RI may include fibromuscular dysplasia or renal artery occlusion following endovascular aortic or renal intervention [19].
In our experience, the importance of close collaboration and clinical comparison among different specialists is the right key to arriving at a clear diagnosis and timely treatment. The nephrologist was contacted by the emergency doctor suspecting pyelonephritis in a patient who did not have renal insufficiency: the analysis and discussion of the images with the interventional radiologist were fundamental to get to a prompt diagnosis and subsequent safe therapeutic procedure.
Results from percutaneous endovascular therapy are not univocal because in literature reports are sporadic and limited only to individual clinical experiences. Available studies and case reports [20-22] are only focused on observational data and show that the efficacy of treatment depends above all on the earliness of the diagnosis, the timeliness of the therapeutic procedure, and the severity of the ischemic lesions (complete or incomplete thrombosis). However, in a small size experience of 14 patients treated with intra-arterial thrombolysis with urokinase, streptokinase, or recombinant tissue plasminogen activator, the authors reported almost 30 years ago a success in 13/14 patients, the only one not-responder because total renal artery occlusion [23]. Delayed diagnosis often leads to giving up the endovascular approach, moreover, the risk of developing acute renal failure is lower the earlier the therapy is started.
The risk of parenchymal damage depends on the type of the vessel involved (main, segmental artery, or subsegmental artery): complete main renal artery occlusion lasting more than 6 hours, or a partial main renal artery occlusion of more than 24 hours duration, above all if there is associated a significant reduction in the kidney function, or in presence of a small kidney, are contraindications to interventional approach.
Ischemia for 4 hours is reported to cause irreversible renal damage [24], however, successful recovery of renal function after prolonged periods of occlusion can be explained by the presence of collateral vessels from lumbar, suprarenal, and ureteral vessels that communicate either perennially into the hilum or internally via arterioles of the capsule [25]. After an extensive discussion with radiologists and interventional cardiologists in our hospital, it was shared a golden time of 24 hours to be considered as a watershed to interventional renal procedures in RI. In case the procedure can be done, intraprocedural heparin administration is to be used.
In our clinical case, the decision to perform angiography was suggested by the need for diagnostic confirmation, and because of the relatively short onset time of the symptoms (less than 24 hours), in order to prevent the development of renal failure due to the loss of renal parenchyma and to potentially recover ischemic nephrotic parenchyma functionality.
Due to the lack of evidence of identified causes for RI and the lack of complete data on a possible thrombophilia, we clinically decided to continue with oral anticoagulant therapy. It would be interesting to have a clinical experience with the use of new anticoagulant drugs in this type of disease.
Conclusion
RI in our experience is more frequent than previously believed and our experience in consecutive case series over a 7-years follow-up shows that renal infarction may well occur even in previously healthy patients of middle age without structural or arrhythmic heart disease, renal impairment, or diagnosis of antiphospholipid antibody syndrome.
Selective arteriography of the renal artery with loco-regional fibrinolysis, executed in our Hospital in 9/24 patients with RI, is a technique that, inexperienced operators, must be taken into consideration in newly occurring RI for both help in diagnosis and its potential efficacy on renal function recovery, without significant collateral effects.
- Bolderman R, Oyen R, Verrijcken A, Knockaert D, Vanderschueren S (2006) Idiopathic renal infarction. Am J Med 119: 356.e9-12 Link: https://bit.ly/3uSuom1
- Paris B, Bobrie G, Rossignol P, Le Coz S, Chedid A, et al. (2006) Blood pressure and renal outcomes in patients with kidney infarction and hypertension. J Hypertens 24: 1649-1654. Link: https://bit.ly/3Brqh1n
- Oh YK, Yang CW, Kim YL, Kang SW, Park CW, et al. (2016) Clinical Characteristics and Outcomes of Renal Infarction. Am J Kidney Dis 67: 243-250. Link: https://bit.ly/36k6TIr
- Eren N, Gungor O, Kocyigit I, Guzel FB¸ Erken E, et al. (2018) Acute renal infarction in Turkey: a review of 121 cases. Int Urol Nephrol 50: 2067-2072. Link: https://bit.ly/3JCbqEx
- Bottomley M J, Gibson M, Alchi B (2019) PR3 vasculitis presenting with symptomatic splenic and renal infarction: a case report and literature review. BMC Nephrol 20: 84. Link: https://bit.ly/3BqSW6I
- Hazanov N, Somin M, Attali M, Beilinson N, Thaler M, et al. (2004) Acute renal embolism. Forty-four cases of renal infarction in patients with atrial fibrillation. Medicine 83: 292-299. Link: https://bit.ly/34ZCSNt
- Karacabey S, Hocagil H, Sanri E, Hocagil AC, Ardic S, et al. (2014) No suspicion, no disease! renal infarction: case series. J Urol 11: 1984-1986. Link: https://bit.ly/3h63mQd
- Chu PL, Wei YF, Huang JW, Chen SI, Chu TS, et al. (2006) Clinical characteristics of patients with segmental renal infarction. Nephrology 11: 336-340. Link: https://bit.ly/3oRqypy
- Bourgault M, Grimbert P, Verret C, Pourrat J, Herody M, et al. (2013) Acute renal infarction: a case series. Clin J Am Soc Nephrol 8: 392-398. Link: https://bit.ly/3I1bCgc
- Hoxie HJ, Coggin CB (1940) Renal Infarction: Statistical study of two hundred and five cases and detailed report of an unusual case. Arch Intern Med 65: 587-594. Link: https://bit.ly/3s1sPAB
- Frost L, Engholm G, Johnsen S, Møller H, Henneberg EW, et al. (2001) Incident thromboembolism in the aorta and the renal, mesenteric, pelvic, and extremity arteries after discharge from the hospital with a diagnosis of atrial fibrillation. Arch Intern Med 161: 272-276. Link: https://bit.ly/3gP2Qpr
- Chondros K, Karpathakis N, Tsetis D, Sofras F, Mamoulakis C (2014) Systemic thrombolysis with the use of tenecteplase for segmental acute renal infarction potentially associated with multiple thrombophilic gene polymorphisms. Hippokratia 18: 67-70. Link: https://bit.ly/36mSAD3
- Bolderman R, Oyen R, Verrijcken A, Knockaert D, Vanderschueren S (2006) Idiopathic renal infarction. Am J Med 119: 356.e9-12. Link: https://bit.ly/3uV9u5M
- Cirillo P, De Rosa S, Pacileo M, Gargiulo A, Leonardi A, et al. (2006) Nicotine induces tissue factor expression in cultured endothelial and smooth muscle cells. J Thromb Haemost 4: 453-458. Link: https://bit.ly/3HSB5Z1
- Csordas A, Bernhard D (2013) The biology behind the atherothrombotic effects of cigarette smoke. Nat Rev Cardiol 10: 219-230. Link: https://bit.ly/36k6R3h
- Chu PL, Wei YF, Huang JW, Chen SI, Chu TS, et al. (2006) Clinical characteristics of patients with segmental renal infarction. Nephrology (Carlton) 11: 336-340. Link: https://bit.ly/3rTWoDK
- Iwasaki M, Joki N, Tanaka Y, Hara H, Suzuki M, et al. (2011) A suspected case of paradoxical renal embolism through the patent foramen ovale. Clin Exp Nephrol 15: 147-150. Link: https://bit.ly/3rQKuuq
- Dao CN, Tobis JM (2011) PFO and paradoxical embolism producing events other than stroke. Catheter Cardiovasc Interv 77: 903-909. Link: https://bit.ly/3uY8RZ8
- Scarpioni R, Michieletti E, Cristinelli L, Ugolotti U, Scolari F, et al. (2005) Atherosclerotic renovascular disease: medical therapy versus medical therapy plus renal artery stenting in preventing renal failure progression: the rationale and study design of a prospective, multicenter and randomized trial (NITER). J Nephrol 18: 423-438. Link: https://bit.ly/3uUu46j
- Silverberg D, Menes T, Rimon U, Salomon O, Halak M (2016) Acute renal artery occlusion: Presentation, treatment, and outcome. J Vasc Surg 64: 1026-1032. Link: https://bit.ly/3petFbp
- Salam TA, Lumsden AB, Martin LG (1993) Local infusion of fibrinolytic agents for acute renal artery thromboembolism: report of ten cases. Ann Vasc Surg 7: 21-26. Link: https://bit.ly/3LCbupk
- Karakurt A (2018) New Thrombolytic Infusion Application of Dissolving Renal Artery Embolic Thrombosis: Low-Dose Slow-Infusion Thrombolytic Therapy. Case Rep Nephrol 2018: 1609025. Link: https://bit.ly/3GQV48X
- Blum U, Billmann P, Krause T, Gabelmann A, Keller E, et al. (1993) Effect of local low-dose thrombolysis on clinical outcome in acute embolic renal artery occlusion. Radiology 1-89: 549. Link: https://bit.ly/3HSGGi4
- Hamilton PB, Phillips RA, Hiller A (1948) Duration of renal ischemia required to produce uremia. Am J Physiol 152: 517-522. Link: https://bit.ly/3rR8ptK
- Koivuviita N, Tertti R, Heiro M, Manner I, Metsärinne K (2014) Thromboembolism as a cause of renal artery occlusion and acute kidney injury: the recovery of kidney function after two weeks. Case Rep Nephrol Urol 4: 82-87. Link: https://bit.ly/3JAWu9D
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
