Archives of Clinical Nephrology
High incidence rate of human herpesvirus 6 infection after Bendamustine, Cytarabine, Etoposide and Melphalan conditioning regimen: A monocentric and retrospective study
Simon Favre1*, Mathieu Sauvezie1, Stéphane Vigouroux2, Reza Tabrizi3, Marie-Sarah Dilhuydy1, Gaelle Laboure4, Margot Robles5, Noel Milpied1 and Kamal Bouabdallah1
2Department of Hematology, CH La Roche sur Yon, La Roche sur Yon, France
3Department of Hematology, Mont de Marsan CH, Mont de Marsan, France
4Department of Hematology, CH Libourne, Libourne, France
5Department of Hematology, Center Hospitalier Territorial de Nouvelle-Caledonie, New Caledonia
Cite this as
: Favre S, Sauvezie M, Vigouroux S, Tabrizi R, Dilhuydy MS (2021) High incidence rate of human herpesvirus 6 infection after Bendamustine, Cytarabine, Etoposide and Melphalan conditioning regimen: A monocentric and retrospective study. Arch Clin Nephrol 7(1): 038-043. DOI: 10.17352/acn.000054Background: Following shortage of Carmustine, BeEAM regimen (Bendamustine, Etoposide, Cytarabine and Melphalan) was used before autologous transplant in relapsed/refractory lymphoma patients. We evaluated safety and efficacy of BeEAM compared to BEAM.
Patients and methods: Ninety consecutive patients receiving BeEAM (30pts) (Bendamustine 100, 120 or 200 mg/m²/d) or BEAM (60 pts) were retrospectively analyzed.
Results: In the BEAM group, 68% had NHL and 32% HL compared to 87% and 13% in the BeEAM group (p = 0,014). Pts were in CR or PR at time of transplant. There was no difference regarding hematologic recovery and transfusion requirements. Highest dose of Bendamustine were associated with grade ≥ 2 kidney toxicity. We observed a significant higher incidence of symptomatic HHV-6 infection (53.3% versus 8.3%), digestive toxicity (36.6% versus 15%) and prolonged hospitalization (25 versus 21 days) with BeEAM. After a median follow up of 61 and 49 months for BEAM and BeEAM, 5y-OS and PFS (76% versus 67% and 56% versus 70%) and TRM (0% versus 3%) were not different.
Conclusions: BeEAM with the highest doses of Bendamustine was associated with increased risk of HHV-6 infection, longer duration of hospitalization, higher rate of digestive toxicity and increased acute kidney failure while survival was comparable.
Introduction
High-dose chemotherapy (HDC) followed by autologous stem cell transplantation (ASCT) is a well-established treatment for patients with relapsed or refractory (R/R) lymphomas after salvage therapy [1-4]. Many HDC regimens with different drug combinations have been used and the BEAM (BCNU/Carmustine, etoposide, cytrabine, melphalan) regimen has become the most commonly used combination before ASCT [5-6]. In France, due to BCNU shortage for several months and based on the results reported by Visani and colleagues [7], Bendamustine was used in combination with Etoposide, Cytarabine and Melphalan (BeEAM) in a new high dose conditioning regimen before ASCT in R/R lymphoma patients. Few studies have been conducted on the feasibility and efficacy of this regimen in patients with aggressive lymphomas. The toxicity profile is similar even though it seems to have a higher incidence of acute kidney failure [8-13]. In this study, we report our experience on the efficacy and safety of BeEAM compared to the classical BEAM regimen before ASCT in R/R lymphoma patients.
Patients and methods
This retrospective and monocentric study was conducted between December 2013 and September 2015. Patients with B or T-cell Non Hodgkin Lymphoma (NHL) and Hodgkin Lymphoma (HL) in complete (CR) or partial (PR) response either after first-line or salvage chemotherapy regimen were included.
All patients were evaluated for response with Computed Tomography (CT) or positron emission tomography (PET) scan before and after ASCT (around day 90), according to Cheson’s 2014 criteria [14].
Patients received HDC with BEAM from December 2013 to January 2015, and BeEAM from February to September 2015 followed by ASCT. BEAM Consisted of Intravenous (IV) BCNU (carmustine) 300 mg/m2 on day −6, Continuous Infusion (CI) of Etoposide 200mg/m2/24h and cytarabine 400mg/m2/24h from day −5 to day −2, and IV Melphalan 140 mg/m2 on day −1. BeEAM consisted of IV Bendamustine 200 mg/m2/d on day −7 and -6; Etoposide, Cytarabine and Melphalan were administered according to the previous schedule. Peripheral Hematopoietic Stem Cells (PHSC) were reinfused on day 0. All patients were monitored for toxicity and complications. Patients with febrile neutropenia were treated with broad-spectrum antibiotics after bacteriological samples.
Platelet and blood transfusions were administered to maintain a hemoglobin level > 8 g/dL and a platelet count > 10 x 109/L. All patients received growth factor support (filgastrim, 5µg/kg/d) starting on day 5 and continuing until neutrophil recovery (ANC > 1000/mm3).
On the basis of our experience regarding HHV-6 infection in the context of ASCT following BEAM conditioning regimen, HHV-6 DNA monitoring was systematically performed by quantitative PCR (qPCR) in whole blood specimens for symptomatic patients with clinical symptoms (e.g. rash, fever) or biological abnormalities and specifically in case of prolonged or recurrent cytopenias. Active and specific HHV-6 infection was defined as 2 consecutive determinations with DNAemias ≥ 450 copies/Ml.
The main objective of this study was to evaluate the safety profile of the two conditioning regimens. Secondary objectives were Event-Free Survival (EFS), Overall Survival (OS), hematologic recovery and amount of blood and platelet units transfused in each group.
Categorical variables were compared using a Pearson’s Chi-scared test and continuous variables were compared using Mann-Whitney test. OS and EFS were estimated using the Kaplan-Meier method and compared using the log rank test. The analyses were performed with SPSS version 12.0.
Results
Patient’s disposition
Ninety patients (M/F ratio = 52/38) were included in this study (BEAM = 60, BeEAM = 30). Median age was 53.8 years in the overall population, 50 years (range: 18.5-66.1) and 56 years (range: 19.9-66.8) in the BEAM and BeEAM groups respectively. There was no statistical difference between the groups in terms of age, sex ratio, number of prior chemotherapy regimens, disease status or renal status at the time of transplant, and median number of CD34+ cells infused.
There were more patients with HL in the BEAM group (30% versus 13.3%, p= 0.014). Among the patients with NHL, there were 35 patients with diffuse large B-cell lymphoma (DLBCL) (22 vs 13), 16 patients with follicular lymphoma (FL) (12 vs 4), 10 pts with mantle-cell lymphoma (MCL) (7 vs 3) and 7 pts with T-cell NHL (1 vs 6). Eighteen patients received HDC in first remission (8 and 10), and 72 in second or subsequent remissions (52 and 20) (47 after a second line (32 and 15) and 25 after 3 or more lines (20 and 5)). More patients in the BEAM group received a platine-based chemotherapy regimen (95% vs 66.7%, p=0.001). Patients in the BeEAM group did not receive the same doses of Bendamustine: the first 6 patients received Bendamustine at a dose of 200mg/m2/day and the dose was then reduced due to a high incidence of acute renal failure. The next 3 patients received 100mg/m2/day with no significant toxicity leading to increase the dose to 120mg/m2/day for the following 21 patients received. The main characteristics of the patients are summarized in Table 1 Figure 1.
Safety and toxicity
Fifty-seven patients in the BEAM group (95%) and all the patients in the BeEAM group experienced neutropenic fever. Microbiological identification was found in 61.6% (n=37/60) and 93.3% (n=28/30) patients respectively. There was no difference between the two groups in the incidence of bacterial infections (56.6% vs 70%, p=NS). However, there was a significant difference in terms of viral infection mainly due to a high incidence of Human-Herpes virus 6 (HHV-6) reactivation in the BeEAM group (16/30 (53.3%) vs 5/60 (8.3%), p < 0.00001). Sixteen patients showed a positive HHV-6 qPCR in the BeEAM group and 10 patients (62.5%) required treatment with foscarnet with a median duration of treatment of 8 days. Six patients did not receive antiviral therapy because of spontaneous resolution of symptoms or cytopenias. There was no significant difference in the occurrence of concomitant serious adverse events or in the number of patients transferred to an intensive care unit between the two groups.
Grade 3 or 4 mucositis were similar between the two groups with 38 patients in the BEAM group (73.1%) and 24 patients (82.5%) in BeEAM group (p = 0.16). Grade 3-4 neutropenic colitis occurred in 9 patients in the BEAM group (15%) and 11 patients in the BeEAM group (36.6%) with a significant difference (p= 0.03). There were no cases of veno-occlusive disease or grade 3 -4 skin toxicity reported.
Acute renal toxicity occurred in 5 patients (8.3%) in the BEAM group (only 1 patient with chronic renal history), and 4 patients (13.3%) in the BeEAM group but this difference was not significant. However, among the 6 patients who received Bendamustine at 200 mg/m2, 3 patients had an acute renal failure. Consequently Bendamustine was reduced to 100 mg/m2 for the following 3 patients where no renal toxicity was observed. Then, the dose of Bendamustine was again increased to 120 mg/m2 (usually used in multiple myeloma) for the remaining 21 patients. Among these last patients, only 1 had a grade 1 acute renal failure. The main non-hematological toxicities are summarized in Table 2.
When analyzing the risk factors for renal failure, we found a strong correlation between patients who received the highest doses of Bendamustine (200 mg/m2) and the risk of acute renal failure compared both to the two others doses (p < 0.00001) and to patients in the BEAM group (p=0.005) Figure 2.
Time for engraftment was similar between the two groups (median absolute neutrophil count (ANC) recovery of 11 days, (9-22) in the BEAM group and (7-19) in the BeEAM group). The median time for platelet recovery (>20 x 109/L) was 12.5 and 12 days for BEAM and BeEAM respectively, p = NS). The median number of red blood cells (RBC) and platelet (PLT) units transfused was similar between the two groups (3 RBC units’ vs 2 and 3 PLT unit’s vs 3.5 in the BEAM and BeEAM groups respectively).
Hospitalization duration was longer in the BeEAM group (median time of 25 days (range 18-59)) than in the BEAM group (median time of 21 days (range 18-32)) (p=0.001).
Two patients died (only in the BEAM group). The first patient died of multiple organ failure secondary to intestinal obstruction with neutropenic colitis on day 9 post-transplant, prior to neutrophil engraftment. The second patient died of sepsis with multi-organ failure (primarily respiratory) on day 42 after transplant.
Response and survival
At the time of transplant, 28 (46.7%) patients were in CR and 32 (53.3%) in PR in the BEAM group. In the BeEAM group, 17 (56.7%) and 13 (43.3%) patients were in CR and PR respectively.
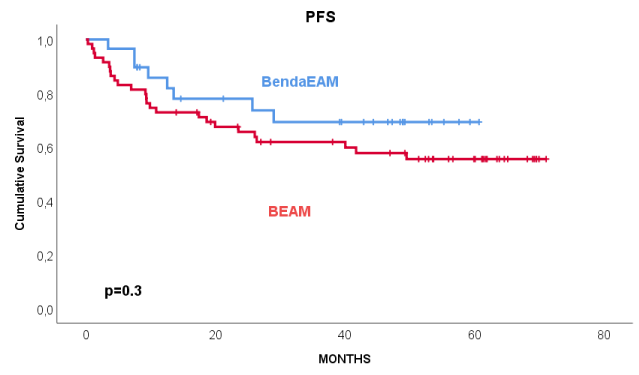
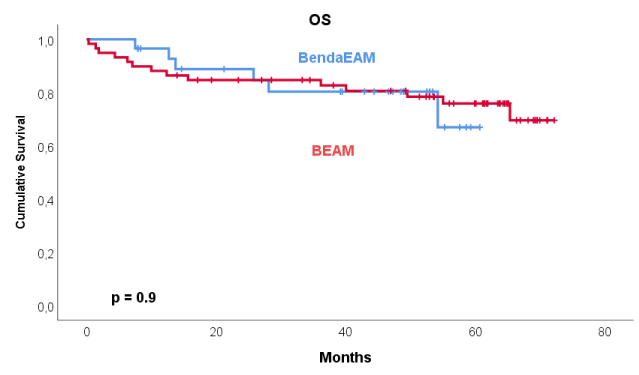
After a median follow-up of 61 months (range: 14-72) in the BEAM group and 49 months (range 8-61) in the BeEAM group after transplantation, the 5 year PFS and OS were not statistically different between the two groups (56% vs 70% for PFS and 76% vs 67% for OS respectively).
Discussion
High-dose chemotherapy followed by ASCT is the standard of care of patients with relapsed/refractory NHL or HL [1-4,15-18]. Several HDC regimens have been used but the BEAM regimen remains the most widely used before ASCT [20,21]. Many studies have been performed in order to compare different HDC regimens and to define the best combination able to target residual malignant cells with the minimal impact on normal tissues and the lowest risk of secondary malignancies [21]. Even broadly used, the BEAM regimen is nevertheless associated with significant side effects during aplasia (severe mucositis, nausea and vomiting, gastrointestinal tract toxicity with severe diarrhea in some cases, hepatotoxicity, nephrotoxicity), leading to a treatment-related mortality (TRM) ranging from 0 to 18% according to studies [6,21-24].
Bendamustine is nitrogen mustard with proven clinical activity in R/R B-cell malignancies, such as indolent lymphomas and CLL [25-29]. The first report by Visani et al, using the BeEAM conditioning regimen (bendamustine at the dose of 200mg/m2, days -7 and -6) in 43 patients with R/R lymphoma (HL, n = 15; NHL, n = 28) resulted in a median PFS of 19 months with a TRM of 0% after a median follow-up of 18 months [7]. These data were confirmed after a longer follow-up of 41 months with a 3-year PFS of 72% and a median OS still not reached [8]. Other studies evaluating the same regimen have shown similar results with 3-year PFS and OS rates of approximately 70% and 85%, respectively [11,12]. In our study, PFS and OS rates were in line with those previously reported.
In our study, we confirm the renal toxicity with acute renal failure occurring in 3 patients among the 6 who received Bendamustine at the dose of 200mg/m2. Acute renal complications after high dose Bendamustine have already been reported in several studies [8-13,30,32-34]. More recently, Chantepie et al identified 3 independent risk factors for acute renal failure after BeEAM: pretransplant chronic renal failure, age > 55 years and Bendamustine dose over 160mg/m2 [31]. Our 3 patients with acute renal failure were all over 55 years of age and 1 of them had chronic renal failure before transplant). Thus, dose reduction of Bendamustine (100 and 120 mg/m2) resulted in a significant decrease in the risk of acute renal failure.
Furthermore, we report a high and significant incidence of active HHV-6 infection in the group of patients receiving the BeEAM conditioning regimen with 53.3% of patients being tested positive compared to13.3% in the BEAM group. HHV-6 infection was systematically investigated in patients with clinical symptoms (rash, fever) or biological abnormalities (prolonged or recurrent cytopenias). HHV-6 is a roseolovirus that is well-known in the exanthema subitum childhood disease with usually favorable outcome. Like other herpsesviruses, HHV-6 has a life-long latency in many organs with a strong tropism for hematopoietic CD34+ progenitor cells in particular. HHV-6 reactivation occurs more commonly in immunocompromised hosts like allogeneic hematopoietic stem cell transplant recipients [35]. A recently published data evaluated prospectively the incidence of HHV-6 infection in patients undergoing ASCT [36]. Among 196 patients, active HHV-6 infection was detected in 22 patients (11.2%) predominantly after BEAM conditioning regimen. These results are in line to those reported in our study where 8.3% of patients in the BEAM group had an HHV-6 infection.
To our knowledge, this high rate of HHV-6 reactivation has not been or very rarely reported in previous studies evaluating the BeEAM regimen, and only in a some patients who did not require anti-viral therapy [31]. Neither has this been reported with Bendamustine in other lymphoid malignancies receiving Bendamustine alone or in association with other cytotoxic agents.
Additionally, several studies showed an increased digestive toxicity mainly with mucositis and colitis after the BeEAM regimen [31,33]. Our study is consistent with these observations with more patients with neutropenic colitis in the BeEAM group.
Finally, we observed prolonged hospitalization duration in the BeEAM group. The length of hospitalization and the differences between both regimens found here are similar to what is reported in the literature [31-37]. This could be explained by the severity of complications (particularly hematological toxicity with delayed ANC and platelets recoveries) together with the need for HHV-6 infection treatment. However, despite prolonged hospitalization, the rate of ICU admission was similar in both groups [30,31].
In conclusion, this study confirms the BeEAM regimen efficacy. However, even if renal toxicity can be avoided by adjusting the dose of Bendamustine, the safety profile of the BeEAM regimen did not appear to be as good as the BEAM regimen due to the high rate of HHV-6 reactivation. Considering the high toxicity rate of Bendamustine which did not translate into a clinical benefit compared to the classical BEAM regimen, we do not recommend the use of BeEAM outside of clinical trials.
- Appelbaum FR (2008) Hematopoietic cell transplantation for non-Hodgkin’s lymphoma: yesterday, today, and tomorrow. J Clin Oncol 26: 2927–2929. Link: https://bit.ly/3wi5PN3
- Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, et al. (2010) Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 28: 4184-4190. Link: https://bit.ly/35kVQeh
- Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, et al. (1995) Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med 333: 1540-1545. Link: https://bit.ly/2U3H0GF
- Isidori A, Piccaluga PP, Loscocco F, Guiducci B, Barulli S, et al. High-dose therapy followed by stem cell transplantation in Hodgkin’s lymphoma: past and future. Expert Rev Hematol 6: 451-464. Link: https://bit.ly/3zoVuRC
- Linch DC, Vaughan Hudson B, Anderson L, Vaughan Hudson G (1997) Impact of high-dose salvage therapy (BEAM) on overall survival in younger patients with advanced large-cell lymphomas entered into BNLI trials. Ann Oncol 8: 63–65. Link: https://bit.ly/3cDNgvb
- Mills W, Chopra R, McMillan A, Pearce R, Linch DC, et al. (1995) BEAM chemotherapy and autologous bone marrow transplantation for patients with relapsed or refractory non-Hodgkin’s lymphoma. J Clin Oncol 13: 588–595. Link: https://bit.ly/3gtWpHH
- Visani G, Malerba L, Stefani PM, Capria S, Galieni P, et al. (2011) BeEAM (bendamustine, etoposide, cytarabine, melphalan) before autologous stem cell transplantation is safe and effective for resistant/relapsed lymphoma patients. Blood 118: 3419–3425. Link: https://bit.ly/3vrNL28
- Visani G, Stefani PM, Capria S, Malerba L, Galieni P, et al. (2014) Bendamustine, etoposide, cytarabine, melphalan, and autologous stem cell rescue produce a 72% 3-year PFS in resistant lymphoma. Blood 124: 3029–3031. Link: https://bit.ly/3xjVHDL
- Visani G, Guidi S, Scalzulli PR, Olivieri A, Angelucci E, et al. (1999) Benda-BEAM highdose therapy prior to auto-SCT is effective in resistant/relapsed DLBCL. Blood 126. Link: https://bit.ly/3cB4xVB
- Noesslinger T, Moestl M, Panny M, Koller E, Keil F, et al. (2014) Autologous stem cell transplantation with Benda-BEAM (Bendamustine, Etoposide, Cytarabine, Melphalan) in aggressive non Hodgkin and Hodgkins lymphoma. Blood 124: 3982-3982. Link: https://bit.ly/3zs0XqH
- Martın A, Redondo AM, Valcarcel D, Gonzalez AP, López-Guillermo A, et al. (2014) A phase 2 study from Spanish Geltamo group investigating the efficacy and safety of bendamustine as part of conditioning regimen for autologous stem-cell transplantation in patients with aggressive lymphomas: second interim analysis. Blood 124: 2524-2524. Link: https://bit.ly/3znf7cI
- Garciaz S, Coso D, Schiano de Collela JM, Broussais F, Stoppa AM, et al. (2016) Bendamustine-based conditioning for non-Hodgkin lymphoma autologous transplantation: an increasing risk of renal toxicity. Bone Marrow Transplant 51: 319-321. Link: https://bit.ly/3iAOP0I
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, et al. (2007) International Harmonization Project on Lymphoma. Revised response criteria for malignant lymphoma. J Clin Oncol 25: 579-586. Link: https://bit.ly/2TUBBl0
- Stiff PJ, Unger JM, Cook JR, Constine LS, Couban S, et al. (2013) Autologous transplantation as consolidation for aggressive non-Hodgkin’s lymphoma. N Engl J Med 369: 1681–1690. Link: https://bit.ly/3zk7vb7
- Crump M, Kuruvilla J, Couban S, MacDonald DA, Kukreti V, et al. (2014) Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J Clin Oncol 32: 3490–3496. Link: https://bit.ly/3cExoIC
- van Imhoff GW, McMillan A, Matasar MJ, Radford J, Ardeshna KM, et al. (2014) Ofatumumab versus rituximab salvage chemoimmunotherapy in relapsed or refractory diffuse large B-cell lymphoma: the Orcharrd study (OMB110928). Blood 124: 630. Link: https://bit.ly/3woqlM2
- Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, et al. (1993) Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet 341: 1051–1054. Link: https://bit.ly/2TUBPIS
- Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, et al. (2002) Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem- cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet 359: 2065–2071. Link: https://bit.ly/3vnSIJ7
- Chen YB, Lane AA, Logan BR, Zhu X, Akpek G, et al. (2015) Impact of conditioning regimen on outcomes for patients with lymphoma undergoing high-dose therapy with autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant 21: 1046–1053. Link: https://bit.ly/3zmMoVx
- Isidoria A, Christofidesb A, Visania G (2016) Novel regimens prior to autologous stem cell transplantation for the management of adults with relapsed/refractory non-Hodgkin lymphoma and Hodgkin lymphoma: alternatives to BEAM conditioning. Leuk Lymphoma 1185785. Link: https://bit.ly/3pNIOPM
- Jo JC, Kang BW, Jang G, Sym SJ, Lee SS, et al. (2008) BEAC or BEAM high- dose chemotherapy followed by autologous stem cell transplantation in non-Hodgkin’s lymphoma patients: comparative analysis of efficacy and toxicity. Ann Hematol 87: 43-48. Link: https://bit.ly/3cEmjHK
- Salar A, Sierra J, Gandarillas M, Caballero MD, Marín J, et al. (2001) Autologous stem cell transplantation for clinically aggressive non- Hodgkin’s lymphoma: the role of preparative regi- mens. Bone Marrow Transplant 27: 405–412. Link: https://bit.ly/3gkH79p
- Mills W, Strang J, Goldstone AH, Linch DC (1995) Dose intensifica- tion of etoposide in the BEAM ABMT protocol for malignant lymphoma. Leuk Lymphoma 17: 263–270. Link: https://pubmed.ncbi.nlm.nih.gov/8580795/
- Fischer K, Cramer P, Busch R, Stilgenbauer S, Bahlo J, et al. (2008) Bendamustine in combination with rituximab (BR) for patients with relapsed chronic lymphocitic leukemia (CLL): a multicenter phase II trial of the German CLL study group. Blood 112.
- Rummel MJ, Al-Batran SE, Kim SZ, Welslau M, Hecker R, et al. (2005) Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin’s lymphoma. J Clin Oncol 23: 3383-3389. Link: https://bit.ly/2TttYlz
- Robinson KS, Williams ME, van der Jagt RH, Cohen P, Herst JA, et al. (2008) Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B-cell and mantle cell non-Hodgkin’s lymphoma. J Clin Oncol 26: 4473-4479. Link: https://bit.ly/3vl1NCv
- Friedberg JW, Cohen P, Chen L, Robinson KS, Forero-Torres A, et al. (2008) Bendamustine in patients with rituximab-refractory indo- lent and transformed non-Hodgkin’s lymphoma: results from a phase II multicenter, single-agent study. J Clin Oncol 26: 204-210. Link: https://bit.ly/35hO5FU
- Leoni LM, Bailey B, Reifert J, Bendall HH, Zeller RW, et al. (2008) Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res 14: 309–317. Link: https://bit.ly/3xejqoI
- Garciaz S, Coso D, Schiano de Collela JM, Broussais F, Stoppa AM, et al. (2016) Bendamustine-based conditioning for non-Hodgkin lymphoma autologous transplantation: an increasing risk of renal toxicity. Bone Marrow Transplant 51: 319-321. Link: https://bit.ly/3iAOP0I
- Chantepie SP, Garciaz S, Tchernonog E, Peyrade F, Larcher MV, et al. (2018) Bendamustine-based conditioning prior to autologous stem cell transplantation (ASCT): results of a French multicenter study of 474 patients from LYmphoma Study Association (LYSA) centers. Am J Hematol 93: 729-735. Link: https://bit.ly/2TqDIgr
- Noesslinger T, Panny M, Simanek R, Moestl M, Boehm A, et al. (2018) High-dose Bendamustine-EAM followed by autologous stem cell rescue results in long-term remission rates in lymphoma patients, without renal toxicity. Eur J Haematol 101: 326-331. Link: https://bit.ly/2SzNblf
- Saleh K, Danu A, Koscielny S, Legoupil C, Pilorge S, et al. (2017) A retrospective, matched pair analysis comparing bendamustine containing BeEAM versus BEAM conditioning regimen: results from a single center experience. Leuk Lymphoma 1-8. Link: https://bit.ly/3xjJHCh
- Gilli S, Novak U, Taleghani BM, Baerlocher GM, Leibundgut K, et al. (2017) BeEAM conditioning with bendamustine-replacing BCNU before autologous transplantation is safe and effective in lymphoma patients. Ann Hematol 96: 421-429. Link: https://bit.ly/3wnRGOr
- de Pagter PJ, Schuurman R, Meijer E, van Baarle D, Sanders EA, et al. (2008) Human herpesvirus type 6 reactivationafter hematopoietic stem cell transplantation. J Clin Virol 43: 361-366. Link: https://bit.ly/3vgckyV
- Balsat M, Pillet S, Tavernier E, Cacheux V, Escuret V, et al. (2019) Human herpesvirus 6 infection after autologous stem cell transplantation: A multicenter prospective study in adult patients. J Infect 79: 36-42. Link: https://bit.ly/3pNS1I1
- Damaj G, Cornillon J, Bouabdallah K, Gressin R, Vigouroux S, et al. (2017) Carmustine replacement in intensive chemotherapy preceding reinjection of autologous HSCs in Hodgkin and non-Hodgkin lymphoma: a review. Bone Marrow Transplant 52: 941-949. Link: https://bit.ly/3zy36l1
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
