Archives of Clinical Nephrology
Evaluating plasma Digoxin concentration after an intravenous loading dose in patients with renal failure
Vahid Eslami1, Fatemeh Mortezapour2, Shiva Samavat3, Shadi Ziae4 and Azin Gheymati5*
2Shahid Beheshti University of Medical Sciences, Cardiovascular Research Center, Tehran, Iran
3Department of Nephrology, Shahid Labbafinejad Medical Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
4Department of Clinical Pharmacy, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran
5Resident of Clinical Pharmacy, School of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
Cite this as
Eslami V, Mortezapour F, Samavat S, Ziae S, Gheymati A (2021) Evaluating plasma Digoxin concentration after an intravenous loading dose in patients with renal failure. Arch Clin Nephrol 7(1): 033-037. DOI: 10.17352/acn.000053Background: Digoxin is a medication of Glycoside family which is commonly prescribed for patients with atrial fibrillation, atrial flutter, and heart failure. With a narrow therapeuticlevel(0.5–2 ng/mL), careful monitoring of digoxin blood level is necessary. Symptoms of digoxin toxicity include nausea, vomiting, visual changes, altered mental status, hyperkalemia, and cardiovascular collapse. As renal failure decreases the clearance of digoxin, and impaired renal function is common in heart failure, this population is at higher risk of toxicity.
Methods & materials: In this prospective study, patients with chronic kidney disease- nondialysis (CKD-ND) who were admitted with heart failure or atrial fibrillation at university hospital, were enrolled. Digoxin-naive patients were treated with a 10 mcg/kg intravenous digoxin loading dose. Serum digoxin level was measured 6 to 12 hours after the last loading dose. Patients were followed for 48 hours for signs of toxicity. Correlation between therapeutic digoxin level and degree of renal failure was evaluated. The effect of serum electrolyte (magnesium, calcium, and potassium) concentration on digoxin level was determined. Pregnant women were excluded from study.
Results: From 2018 to 2020, 87 CKD patients, (60 (69%) men and 27 (31%) women) aged from 31 to 92 years old, with a mean age of 70.51 ± 14.06 years were admitted to the cardiac unit. Near 80% of the cohort were CKD stage 3 and 4 patients. About half of patients had digoxin levels in therapeutic range (45 cases = 51.7%), followed by 24.1% with supra therapeutic and the other 24.1% with toxic levels. There was significant relationship between GFR and serum digoxin concentration (p-value = 0.038); the lower the GFR was, the higher was the digoxin level and also between serum creatinine and digoxin level (p value: .04). Serum digoxin concentration had significant correlation with serum magnesium level (p-value = 0.006).
Conclusion: this study demonstrated that monitoring plasma Digoxin concentration after an intravenous loading dose in patients with renal failure can be very helpful to prevent digoxin toxicity. The lower the GFR was, the higher the digoxin level and the risk for toxicity if the bolus and even maintenance do not monitor meticulously.
Introduction
Digoxin is one of the frequently prescribed medications with a narrow therapeutic range and many drug interactions [1,2]. Increasing cardiac output and thereby decreasing ventricular filling pressures are the potential benefits of digoxin therapy in heart failure patients as a positive inotropic agent [3]. Plasma half-life range of digoxin varies from 20 to 50 hours, which is variable with a bioavailability of approximately 66%. Steady state plasma concentrations of digoxin are also altered proportionally to renal clearance of creatinine. The bioavailability and biotransformation of digoxin do not seem to vary between healthy subjects and patients with renal insufficiency. As the volume of distribution is smaller in patients with severe renal failure that normal subjects, the loading dose has to be altered. On the basis of this assumption, with decreasing creatinine clearance, the total body clearance as well as the renal clearance of digoxin is reduced [4].
In patients with renal failure, the half-life of digoxin increases, so that even in end-stage renal disease, it can be up to 4-6 days [5]. Due to the renal excretion of digoxin, renal dysfunction can lead to higher plasma concentrations and consequent toxicity. Renal failure, congestive heart failure, old age, and electrolyte abnormalities such as low potassium, phosphate, or calcium may worsen the condition [6].
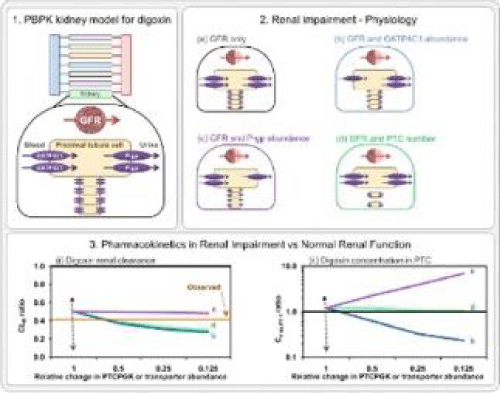
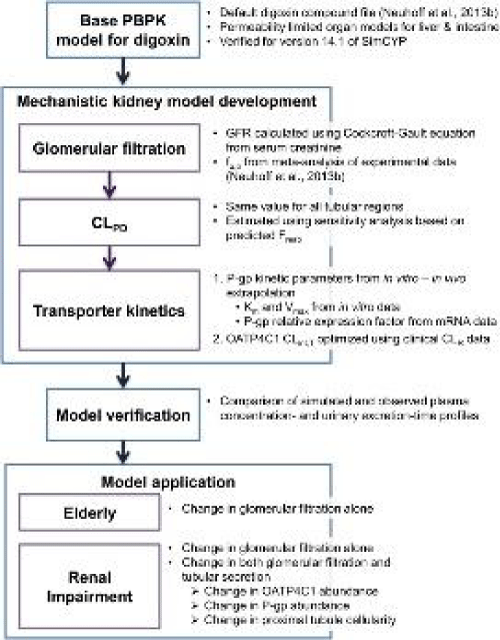
Glomerular filtration damages in Chronic Renal Failure (CRF) constantly increase the risk of atherosclerosis and other heart diseases [7]. Oxidative stress also plays a central role in cardiovascular dysfunction. Evidence shows that uremia and hemodialysis in CRF increase levels of reactive oxygen species [8,9]. Thus, renal dysfunction is common in heart failure patients and can increase related mortality especially in GFR<60 ml/min [10]. Optimum Serum digoxin concentration (SDC) is supposed to be about 0.8 ng/mL, and it is essential to monitor its plasma concentration, particularly when ejection fraction falls below45% [11,12]. Since Digoxin inhibits the sodium-potassium pumps and increases intracellular calcium in myocardial cells, patients with renal dysfunction are more prone to electrolyte imbalance in case of taking Digoxin [13]. Digoxin toxicity symptoms includes nausea, vomiting, visual changes, altered mental status, hyperkalemia, and cardiovascular collapse[14]. The mechanism and physiologically based pharmacokinetic of digoxin in renal failure are shown in the figures below (Figures 1, 2) [15].
Perceiving renal function during the treatment of heart failure using digoxin is necessary to prevent possible complications [16]. The purpose of this study is to assess the plasma Digoxin concentration after an intravenous loading dose in patients with renal failure. Also, this study evaluates the relationship between serum digoxin level with demographic and clinical characteristics including age, sex, serum electrolyte values (sodium, potassium, and magnesium), and creatinine level.
Material and methods
This prospective study was conducted in patients with Heart Failure (HF) or Atrial Fibrillation (AF) with underlying chronic kidney dysfunction who referred to the university hospital, from 2018 to 2020. In this study, 87 patients aged 31 to 92 years were hospitalized in the cardiac care unit and after obtaining informed written consent, they were included in the study. All the study protocols were approved by the Ethics Committee of Shahid Behesti University of Medical Sciences. All the information was recorded in patients’ electronic record.
Patients who had not consumed digoxin within 2 weeks before the loading dose and had received digoxin at a loading dose of 10 mcg/Kg (half of the total dose [5mcg/Kg] prescribed upon presentation and the resting half as aquarter [2.5 mcg/dl] atevery 6hours) were included. Blood sample was drawn to assess digoxin concentration 6 to 12h after last digoxin administration. Patients with hypothyroidism (hypothyroidism or hyperthyroidism), renal replacement therapy, digoxin use in the last two weeks, acute renal failure, concomitant use of amiodarone, calcium channel blockers, quinine, quinidine, macrolides and cyclosporine consumption, weight over 120 kg, age under 18, pregnant women, death during the study and dissatisfaction with the study were excluded.eGFR was calculated based on Cockcroft-Gault equation and lean body weight was considered in it. Patients were categorized into 4 groups according to GFR, including stage 2 (GFR>60), stage 3 (GFR: 30-60), stage 4 (GFR: 15-30) and stage 5-nondialysis (GFR<15). Serum Digoxin level under 1.2 nmol/L was considered as therapeutic, between 1.2 to 2 nmol/L as Supra therapeutic, and above 2 nmol/L as toxic.
Patients were observed for 48 hours after receiving Digoxin loading dose since toxicity symptoms (including anorexia, nausea, vomiting, weakness, visual disturbances, or sinus bradycardia [< 60 bpm]) may occur if serum digoxin concentration increases to 2 nmol/L or above.
Demographic and clinical characteristics including age, sex, serum electrolyte values (sodium, potassium, and magnesium), and creatinine level were also recorded.
Statistical analysis
All variables were recorded in Statistical Package for the Social Sciences (SPSS), Version 25.0. Statistical Analysis was performed using the Pearson correlation. Statistical significance was considered as p ≤0.05 and the results were presented by using tables of distribution, frequency and percentages for categorical variables. Kai square, Fisher, T-test and independent multivariate analysis were used.
Results
During the study period, 87 patients aged 31 to 92 years, with a mean age of 70.51±14.06. yearswere enrolled. The patients consist of 60 (69%) men and 27 (31%) women. The most common underlying cardiac disorder was heart failure (60 patients, 69%), followed by AF (18 patients, 20.7%) and concomitant AF and HF (9 patients, 10.3%).
Mean GFR of patients was 28.86±14.16ml/min (ranged 10 to 70 ml/min). Also, meanserum digoxin concentration level after loading dose was 1.48±0.99ml/min (ranged 0.2 to 4.56ml/min). There was a significant correlation between GFR and serum digoxin concentration (p-value = 0.03). Also, a significant correlation between serum creatinine and digoxin level was observed (p-value =0.04).
Patients were categorized into 4 groups according to GFR including stage 2 (6 patients = 6.9%), stage 3 (45 patents = 51.7%), stage 4 (24 patients = 27.6%) and stage 5 (12 patients = 13.8%). The mean serum digoxin concentration was measured as follows: stage 2 (0.65 ± 0.16 ngr/ml), stage 3 (1.44 ± 0.63 ngr/ml), stage 4 (1.73 ± 1.43 ngr/ml) and stage 5 (1.57 ± 1.17 ngr/ml).
There was a significant correlation between serum digoxin concentration and serum magnesium level (2.09 ± 0.30 meq/L)(p-value = 0.006), but other demographic and clinical characteristic including age, sex, GFR, serum sodium, potassium and creatinine values did not show any correlation with serum digoxin concentration Table 1
Serum digoxin level was in therapeutic range in almost half of the patients (45 cases = 51.7%), while21 cases (24.1%) had supra therapeutic levels and toxic levels was observed in 21 cases (24.1%). Laboratory findings of different groups and correlation with Digoxin therapeutic concentration are shown in the Table 2. There was no significant differences between clinical signs of digoxin toxicity like nausea and vomiting between groups.
Discussion
Our study shows a significant correlation between the measured digoxin serum concentration with GFR (P value= 0.03) and Creatinine (p value= 0.04), while no correlation was found with age and gender. It was also shown that although there was a significant correlation between digoxin levels and magnesium and sodium levelin two groups , correlations with different stages of renal function (GFR) were not observed. These results are the same as reported in similar studies [17,18].
Digoxin, as a digitalis glycoside, improves the hemodynamic and neurohormonal perturbations which plays a crucial role in Heart Failure (HF) induced renal dysfunction. Abnormal energy metabolism increased production of reactive oxygen species (ROS), and defects in excitation-contraction are the hallmarks of Heart Failure due to impaired ventricular filling or blood ejection[19,20]. Digoxin increases intracellular calcium in myocardial cells and inhibits the sodium-potassium pump, besides, induces an increase in intracellular Na+ and Ca2+ [21]. However, our study did not show a significant relationship between sodium and potassium levels with digoxin levels, which may be due to minor changes in the levels of electrolytes. However, the difference in sodium levels between the two groups with Digoxin therapeutic and toxicity concentration, was significant.
A case-control study of patients who took digoxin for a long time and had a toxic level compared with those who recently received a therapeutic dose of digoxin reported that There were no differences between case and control groups with creatinine, age, or sex [22].
The findings show that there is a significant direct link between magnesium and digoxin. These results were inconsistent with the 1991 study by Young IS et al that examined the relationship between serum magnesium levels and digoxin toxicity. Results demonstrated that magnesium deficiency was the most common electrolyte disorder associated with digoxin toxicity [23].
The results of this study show the importance of monitoring patients receiving a loading dose of digoxin and have renal dysfunction. About 30 to 50% of Patients with heart failure suffer from kidney dysfunction. Inflammation, oxidative stress, impaired hydro saline homeostasis, and diuretic resistance are common mechanisms in heart failure and kidney dysfunction which lead to worsen diseases prognosis [24]. In Testani JM, et al study, patients who suffered from impaired renal failure, digoxin was associated with survival benefit in patients significantly (adjusted hazard ratio=0.49, 95% CI 0.3–0.8; P = .006; P interaction = .026) [25]. Also The results of a study by Voors, AA, et al. in 2011 showed renal dysfunction with decreased glomerular filtration rate and increased serum creatinine, raised mortality and hospitalization in patients with cardiac disease and increased sodium and fluid retention also induced resistance to loop diuretics [26]. While heart failure progresses, decrease arterial pressure combined with an increase in venous pressure leads to glomerular filtration declines. Preventing the development of kidney damage in patients with cardiac disorders is a major challenge [27]. The glomerular filtration rate (GFR), serum electrolyte values and Creatinine levels are used to monitor chronic kidney disease [28]. GFR less than 10.0 ml per minute per 1.73 m2 considers as end-stage renal disease and they are higher risk for cardiovascular events compared to other stages [29]. A study in 2018 introduced urea and serum creatinine as the best criteria for predicting mortality in patients with chronic digoxin poisoning [30].
A study in California of 41 patients receiving digoxin with blood urea nitrogen (RUN), 26.1 ± 12.8 mg per 100 ml; creatinine, 1.1 + 0041 mg per 100 ml; creatinine clearance, 78 ± 42 ml/min/1.73 m2; digoxin clearance, 66.6 ± 42.1 ml/min/1.73 m2 indicated that digoxin has some degree of tubular reabsorption in addition to filtration and secretion [31]. Another study was done on 124 patients ranging in age from 22 to 88 years with serum creatinine ranged from 0.40 to 1.80 mg/dL (median 0.90 mg/dL, IQR 0.79, 1.10) and SDC was 1.12±0.34 mcg/L showed importance Prediction Of Serum Digoxin Concentration Using Estimated Glomerular Filtration Rate [32]. Drugs including amiodarone, verapamil, diltiazem, nifedipine, quinidine, quinine, clarithromycin, azithromycin, and erythromycin, tetracycline, and cyclosporine could influence digoxin concentration [33]. Hypokalemia, hypomagnesemia, hypercalcemia, myocardial ischemia, hypoxemia, and acid-base disturbances are conditions that increase the serum digoxin concentration which may cause toxicity. Long-term therapy with digoxin can lead to overdose and emergent condition [34,35]. Bradycardia and life-threatening ventricular arrhythmias are results of Digoxin toxicity. According to this, close observation in patients with Atrial Fibrillation or Heart Fibrillation and CKD is recommended [36]. The results of these studies confirm the result of the present study.
Our prospective study has some limitations because it was a Hospital-Based Study with a limited sample size and some of the data like causes of «toxic» digoxin concentrations are not available.
Conclusion
This study emphasize on importance of weight adjusted doseselection ofdigoxin not only in the maintenance dose but also in the bolus dose and demonstrated that monitoring plasma Digoxin concentration after an appropriate and measured intravenous loading dose in patients with renal failure can be very helpful to prevent digoxin toxicity. By checking the serum level of digoxin during treatment and the level of serum electrolytes, including magnesium, it can be ensured that no digoxin poisoning will occur, and in cases with digoxin toxic symptoms, the necessary procedures are taken.
- Williamson KM, Thrasher KA, Fulton KB, LaPointe NM, Dunham GD, et al. (1998) Digoxin toxicity: an evaluation in current clinical practice. Arch Intern Med 158: 2444-2449. Link: https://bit.ly/34DS4fx
- Englund G, Hallberg P, Artursson P, Michaëlsson K, Melhus H (2004) Association between the number of coadministered P-glycoprotein inhibitors and serum digoxin levels in patients on therapeutic drug monitoring. BMC Med 2: 8. Link: https://bit.ly/3wTzLPs
- Gheorghiade M, Ferguson D (1991) Digoxin. A neurohormonal modulator in heart failure?. Circulation 84: 2181-2186. Link: https://bit.ly/3c8PL8E
- Vöhringer HF, Rietbrock N (1981) Digitalis therapy in renal failure with special regard to digitoxin. Int J Clin Pharmacol Ther Toxicol 19: 175-184. Link: https://bit.ly/2S6QSi5
- Yang EH, Shah S, Criley JM (2012) Digitalis toxicity: a fading but crucial complication to recognize. Am J Med 125: 337-343. Link: https://bit.ly/3wKkTTA
- Dattoma L, Shah J (2019) Digoxin Toxicity Presenting as “Stomach Upset” and “Fatigue”. Proceedings of UCLA Health 23. Link: https://bit.ly/3yUo4tO
- Durmaz I, Büket S, Atay Y, Yağdı T, Özbaran M, et al. (1999) Cardiac surgery with cardiopulmonary bypass in patients with chronic renal failure. J Thorac Cardiovasc Surg 118: 306-315. Link: https://bit.ly/3wRwBfq
- Clermont G, Lecour S, Lahet JJ, Siohan P, Vergely C, et al. (2000) Alteration in plasma antioxidant capacities in chronic renal failure and hemodialysis patients: a possible explanation for the increased cardiovascular risk in these patients. Cardiovasc Res 47: 618-623. Link: https://bit.ly/3fJzRUe
- Nogueira FN, Romero AC, da Silva Pedrosa M, Ibuki FK, Bergamaschi CT (2020) Oxidative stress and the antioxidant system in salivary glands of rats with experimental chronic kidney disease. Arch Oral Biol 113: 104709. Link: https://bit.ly/3g1FBb1
- Shlipak MG, Smith GL, Rathore SS, Massie BM, Krumholz HM (2004) Renal function, digoxin therapy, and heart failure outcomes: evidence from the digoxin intervention group trial. J Am Soc Nephrol 15: 2195-2203. Link: https://bit.ly/3yOYB4W
- Rathore SS, Curtis JP, Wang Y, Bristow MR, Krumholz HM (2003) Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA 289: 871-878. Link: https://bit.ly/34F0MKC
- Rathore SS, Wang Y, Krumholz HM (2002) Sex-based differences in the effect of digoxin for the treatment of heart failure. N Engl J Med 347: 1403-1411. Link: https://bit.ly/2RUgOxz
- Mutlu M, Aslan Y, Kader Ş, Aktürk-Acar F, Dilber E (2019) Clinical signs and symptoms of toxic serum digoxin levels in neonates. Turk J Pediatr 61: 244-249. Link: https://bit.ly/2ST16m8
- Bridwell RE, Baker KA, Hoyte CO, Ng PC (2019) Digoxin Toxicity in a Patient with Pacemaker: A Case Report. Cureus 11: e6056. Link: https://bit.ly/3fI1DAm
- Scotcher D, Jones CR, Galetin A, Rostami-Hodjegan A (2017) Delineating the role of various factors in renal disposition of digoxin through application of physiologically based kidney model to renal impairment populations. J Pharmacol Exp Ther 360: 484-495. Link: https://bit.ly/3peoerm
- Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, et al. (2013) Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 310: 2533-2543. Link: https://bit.ly/2S6252z
- Ooba N, Sente A, Abe M, Watanabe F, Tsutsumi D, et al. (2020) Frequency of Clinical Monitoring of Serum Concentrations of Digoxin, Potassium, and Creatinine, and Recording of Electrocardiograms in Digoxin-Treated Patients: A Japanese Claims Database Analysis. Biol Pharm Bull 43: 913-916. Link: https://bit.ly/3fHPagn
- Vazquez-Hernandez M, Bouzas L, Tutor JC (2009) Glomerular filtration rate estimation using the Cockcroft-Gault and modification of diet in renal disease formulas for digoxin dose adjustment in patients with heart failure. Ups J Med Sci 114: 154-159. Link: https://bit.ly/3wQQ9At
- Testani JM, Brisco MA, Tang WW, Kimmel SE, Tiku-Owens A, et al. (2013) Potential effects of digoxin on long-term renal and clinical outcomes in chronic heart failure. J Card Fail 19: 295-302. Link: https://bit.ly/3cdY7vH
- Li X, Xu G, Wei S, Zhang B, Yao H, et al. (2019) Lingguizhugan decoction attenuates doxorubicin-induced heart failure in rats by improving TT-SR microstructural remodeling. BMC Complement Altern Med 19: 360. Link: https://bit.ly/3uJlBiG
- Bauman JL, DiDomenico RJ, Galanter WL (2006) Mechanisms, manifestations, and management of digoxin toxicity in the modern era. Am J Cardiovasc Drug 6: 77-86. Link: https://bit.ly/34E8WTt
- Manini AF, Nelson LS, Hoffman RS (2011) Prognostic utility of serum potassium in chronic digoxin toxicity. Am J Cardiovasc Drugs 11: 173-178. Link: https://bit.ly/3fGvkSl
- Young IS, Goh EM, McKillop UH, Stanford CF, Nicholls DP, et al. (1991) Magnesium status and digoxin toxicity. Br J Clin Pharmacol 32: 717-721. Link: https://bit.ly/34CA8Cf
- Ruocco G, Palazzuoli A, TerMaaten JM (2020) The role of the kidney in acute and chronic heart failure. Heart Fail Rev 25: 107-118. Link: https://bit.ly/3wQrgoC
- Testani JM, Brisco MA, Tang WW, Kimmel SE, Tiku-Owens A, et al. (2013) Potential effects of digoxin on long-term renal and clinical outcomes in chronic heart failure. J Card Fail 19: 295-302. Link: https://bit.ly/3cdY7vH
- Voors AA, Dittrich HC, Massie BM, DeLucca P, Mansoor GA, et al. (2011) Effects of the adenosine A1 receptor antagonist rolofylline on renal function in patients with acute heart failure and renal dysfunction: results from PROTECT (placebo-controlled randomized study of the selective A1 adenosine receptor antagonist roloff sylline sess treatment effect on congestion and renal function). J Am Coll Cardiol 57: 1899-1907. Link: https://bit.ly/3fGbKpq
- de Silva R, Nikitin NP, Witte KK, Rigby AS, Goode K, et al. (2006) Incidence of renal dysfunction over 6 months in patients with chronic heart failure due to left ventricular systolic dysfunction: contributing factors and relationship to prognosis. Eur Heart J 27: 569-581. Link: https://bit.ly/34FkJRG
- Filippatos G, Farmakis D, Parissis J (2014) Renal dysfunction and heart failure: things are seldom what they seem. Eur Heart J 35: 416-418. Link: https://bit.ly/3x4iqDV
- Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, et al. (2004) Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med 351: 1285-1295. Link: https://bit.ly/3icYdYi
- Ekinci MD, Kilic TY (2018) Serum Urea and Creatinine Levels are Better Predictors of Mortality than Serum Potassium Levels in Chronic Digoxin Toxicity Link: https://bit.ly/2RigAjs
- Halkin H, Sheiner LB, Peck CC, Melmon KL (1975) Determinants of the renal clearance of digoxin. Clin Pharmacol Ther 17: 385-394. Link: https://bit.ly/3fI4dq2
- Sae-lim O, Doungngern T, Jaisue S, Cheewatanakornkul S, Arunmanakul P, et al. (2019) Prediction of Serum Digoxin Concentration Using Estimated Glomerular Filtration Rate In Thai Population. Int J Gen Med 12: 455-463. Link: https://bit.ly/2TBKcsT
- Pawlosky N, MacDonald E, Patel R, Kanji S (2013) Evaluation of digoxin concentration after loading dose in patients with renal dysfunction. Can J Hosp Pharm 66: 104-109. Link: https://bit.ly/3cbfSf6
- Pincus M (2016) Management of digoxin toxicity. Aust Prescr 39: 18-20. Link: https://bit.ly/3ccl2HI
- Caspi O, Zylber-Katz E, Gotsman O, Wolf DG, Caraco Y (1997) Digoxin intoxication in a patient with end-stage renal disease: efficacy of digoxin-specific Fab antibody fragments and peritoneal dialysis. Ther Drug Monit 19: 510-515. Link: https://bit.ly/3yUOgEK
- Shin JH, Kang KW, Kim JG, Lee SJ (2018) Concurrent renal dysfunction with ischemic heart disease is an important determinant for cardiac and cerebrovascular mortality in patients on chronic digoxin therapy for atrial fibrillation. Kidney Res Clin Pract 37: 130-137. Link: https://bit.ly/3c6Pnr9
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
