Archives of Clinical Nephrology
Crystalline nephropathy due to adenine phosphoribosyl transferase deficiency as a cause of renal allograft dysfunction
Umapati Hegde1*, Rajapurkar Mohan2, Gang Sishir3, Amit Jojera4 and Shailesh Soni4
2Director Postgraduate services and Research, Department of Nephrology, Muljibhai Patel Urological Hospital, Nadiad, Gujarat-387001, India
3Chairman, Department of Nephrology, Muljibhai Patel Urological Hospital, Nadiad, Gujarat-387001, India
4Consultant Pathologist, Department of Pathology, Muljibhai Patel Urological Hospital, Nadiad, Gujarat-387001, India
Cite this as
Hegde U, Mohan R, Sishir G, Jojera A, Soni S (2021) Crystalline nephropathy due to adenine phosphoribosyl transferase deficiency as a cause of renal allograft dysfunction. Arch Clin Nephrol 7(1): 001-005. DOI: 10.17352/acn.000049APRT deficiency is a rare but under recognized genetic disease.
Recurrent urolithiasis and DHA nephropathy are the two clinical manifestations of APRT deficiency and diagnosis can be made at any age and recurs after renal transplant. Allopurinol is the cornerstone in preventing recurrence.
APRT activity assay and genetic testing are useful for confirmation of diagnosis, for family screening and in difficult cases of urolithias or crystalline nephropathy.
Background
Adenine Phosphoribosyl Transferase (APRT) deficiency is an inherited condition presenting from infancy to late adulthood. A common feature is recurrent kidney and urinary tract stones and obstructive symptoms. It presents as radiolucent nephrolithiasis or Tubular crystal deposition can occur associated with marked interstitial fibrosis. The stones are characteristically radiolucent and they can recur following renal transplant. 2,8 Dihydroxy adenine (DHA) formation is blocked by Xanthine Oxidase blocker allopurinol reduces interstitial fibrosis [1]. DHA-induced nephropathy may recur in the allograft and it is diagnosed only when the allograft failure has been investigated more carefully. We here report a unique case of recurrence of crystalline nephropathy due to APRT deficiency causing renal allograft dysfunction.
Case summary
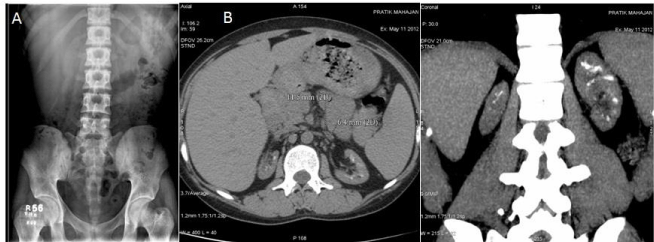
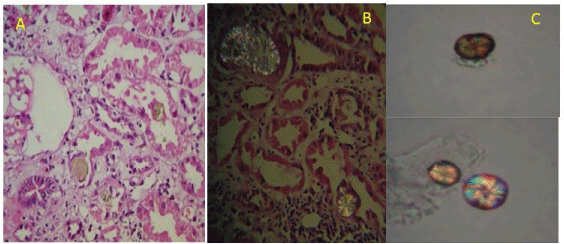
A 22-year-old male born of non-consanguineous marriage, End stage renal failure on dialysis presented with the history of burning sensation during micturition and suprapubic pain at the age of 3 years. At the age of 9-10 years he had burning micturition and difficulty in passing urine, diagnosed as bladder stones and urethral stones. He underwent surgical removal of stones and the stone was detected to be of calcium oxalate. He had lithuria and bladder stone removal was done at the age of 12 years. He had decreased appetite, loss of taste, weakness, anemia, drowsiness, was unable to concentrate and the condition was diagnosed as chronic kidney disease. At the age of 20 years, he was initiated on HD through right IJV DLC in view of uremic convulsion. Later left RC AVF was constructed which had primary failure and then left BC AVF which was his access when he came to our hospital. He was detected to be hepatitis C positive (Genotype 3) with normal liver function so he was treated with injection. Interferon thrice per week for 12 months. His X ray KUB didn`t show any stone but CT KUB showed bilateral small kidneys with multiple soft calcific density foci in renal parenchyma (Figure 1) In view of history of stone disease, he underwent 24-hour urinary metabolic evaluation which was not suggestive of hyperuricosuria. As the stone was radiolucent, oxalosis was excluded and genetic work up was deferred. His 55-year-old father come forward as prospective donor and found suitable for kidney donation. He underwent ABO compatible live renal transplant with triple immunosuppressant comprising of prednisolone, mycophenolate mofetil and cyclosporine without induction. There was immediate graft function and the Creatinine decreased to 1.57 mg/dl on the fourth day. However, there was rise in Creatinine to 1.78 mg/dl with cyclosporine level of 120ng/ml. His Cyclopsorine dose was increased. However, in view of rising Creatinine, graft biopsy was done on 12th post-operative day, which showed borderline rejection (interstitial inflammation with occasional Tubulitis). Inj. Methylprednisolone 250mg each was given for 3 days. His creatinine decreased to 1.69mg/dl with adequate urine output and he was discharged on 14th post-op day. During the OPD visits every alternate day, there was again serial rise in creatinine to 1.84mg/dl and hence repeat graft biopsy was performed on 25th day post-transplant, which showed patchy infiltrate in interstitium and mild edema with tubular vacuolization and crystal deposits (Figure 2). There was no evidence of tubulitis/glomerulitis/endothelitis. His urine examination showed typical maltese crossed 2,8 dihydroxy adenine crystal (Figure 2). His blood and urine samples were sent for APRT enzyme analysis and crystal detection to St. Thomas hospital, London. His APRT activity was <2nmol/h/mg HB (normal range--16-32nmol/h/mg HB). He was started on allopurinol in view of APRT deficiency. On serial follow up his creatinine remained between 1.9-2.1mg/dl.
Discussion
2, 8-Dihydroxy adenine urolithiasis or crystalline nephropathy is the result of enzyme deficiency, adenine phosphoribosyl transferase (APRT), a salvage enzyme present in all human cells for purine metabolism [2]. It catalyzes the formation of adenine monophosphate from adenine in the presence of phosphoribosyl pyrophosphate. In the absence of APRT, adenine is oxidized by xanthine oxidase to 2, 8-dihydroxyadenine via the intermediate, 8-hydroxyadenine. 2,8-Dihydroxyadenine is poorly soluble in any urinary pH, resulting in 2, 8- dihydroxyadenine stones, or precipitate in the lumen and deposition in the epithelial cells and interstitium resulting in crystalline nephropathy [3]. We describe a case of recurrent bladder stone disease and radiolucent stones in the kidney causing End stage renal failure, he underwent live related renal transplant. He had acute graft dysfunction, graft biopsy showed interstitial inflammation and crystalline nephropathy consistent with 2,8 DHA. The diagnosis of APRT deficiency was made only after renal transplantation. The APRT deficiency was confirmed by the typical crystal in urine examination and the measurement of enzymatic activity in the Red blood cells.
In 1968, Kelley, et al. [4] Identified partial Adenine Phosphoribosyltransferase (APRT) deficiency of autosomal inheritance. Features and severity vary greatly in affected individuals, even among the family members. Urolithiasis is the most common manifestation of APRT deficiency in both children and adults. DHA nephropathy represents the second common manifestation, typically occurs in patients who have remained undiagnosed and untreated despite a history of recurrent urolithiasis. Imaging studies demonstrating the absence of stone do not definitively rule out the possibility of DHA nephropathy [5]. 15% of the patients develop End stage renal failure [6] and they are diagnosed APRT deficiency only after renal transplantation. 15-20% of people with APRT deficiency present no symptoms. Although APRT deficiency has no known extrarenal manifestations, in some patients suggested partial APRT deficiency could contribute to hyperuricemia and gout. Although the worldwide prevalence of APRT deficiency is largely unknown, it has been estimated to be 1/100,000 in Caucasian population, 1: 27000 people in Japan, rarer in Europe 1: 50000-100000 people [7]. Inheritance is autosomal recessive typically involving Chromosome 16q24 and has presently 24 functional known mutations [7].
APRT deficiency may present at any age ranging from infancy to more than 70 years of age. In a French series only 37% of individuals with APRT deficiency were diagnosed before age 16 years. Median age is 37 years. Common feature is recurrent kidney and urinary tract stones and obstructive symptoms. In infancy presents as tiny ravel of reddish brown material in baby diapers. Renal functions affected by early to late adulthood. 60-75% patients are affected in adulthood. The presenting symptoms are stones, urinary tract infections, hematuria, abdominal pains and nausea and vomiting, nearly 10% finally progress towards ESRD before the diagnosis.
More than 400 subjects with APRT deficiency have been reported in the medical literature including case reports, case series, and registry data [6]. A study of 67 patients from 56 families by Irène Ceballos-Picot etal [8] from Necker Universitary Hospital, Paris, identified complete APRT deficiency 73% presented with recurrent nephroloithiasis and due to misdiagnosis, 20% cases developed irreversible loss of renal function. In a registry data consisting of 53 patients by Runolfsdottir HL, et al. [9] with APRT deficiency were followed up for median duration of 10.3 years. At the end of the study 42% patients were in CKD 3-5 stage. There was slower fall in the median eGFR -0.38 mL Vs -5.74/ml/min/1.73m2 per year in patients receiving treatment with Allopurinol compared to those not treated prior to the development of stage 5 CKD. In another study by Runolfsdottir HL, et al. showed that if the therapy is initiated early in the course the eGFR is maintained at higher range (children - 114 (70-163) Vs adults - 62 (10-103) mL/min/1.73 m2) [10].
In renal transplantation the absence of prophylactic treatment, 2,8-DHA crystalline nephropathy can recur in the renal allograft, leading to allograft loss in more than 25% of cases. Recurrence of nephrolithiasis has also been reported but is less common in transplant than in native kidneys. 2,8-DHA crystals can be detected in the urine within the first few days after renal transplantation, leading to delayed graft function and primary graft non-function. The factors underlying variable presentation are unclear. The environmental factors such as fluid intake, acute episodes of dehydration, and purine intake influence the phenotypic variability. A study of recurrence after transplant by Zaidan M, et al. [11] Consisting of 9 patients with recurrent 2,8-DHA crystalline nephropathy,5 had acute on chronic graft dysfunction, 1 acute graft dysfunction and 1 had delayed graft dysfunction at the timer of diagnosis. With Allopurinol therapy graft function improved in 7 patients, stabilized in 1 and worsened in one patient. In our patient also had acute on chronic graft dysfunction and stabilized with allopurinol therapy.
There are 2 types of deficiencies in enzyme: APRT 1 deficiency: Absent enzyme levels (complete deficiency in Vivo and in Vitro), usually found in Caucasians populations with 0.4-1.1% distribution in general population. APRT 2 deficiency: Decreased levels of enzyme by less than 30% (complete deficiency in vivo but partial deficiency in vitro) usually found in the Japanese with 05-1.2% distribution in general population amounts to 70% of the cases.
Detection of APRT deficiency
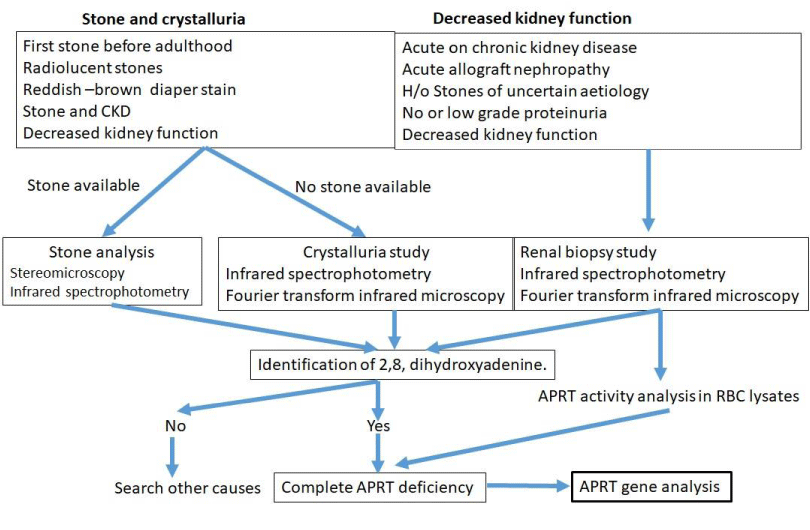
The identification of DHA in stone or urine is pathognomonic of APRT deficiency. The diagnosis of APRT deficiency disease in our patient was confirmed by: (i) the absence of APRT enzyme activity in erythrocyte lysates by High Performance Liquid Chromatography (HPLC) (ii) the characterization of 2,8- DHA crystals in the urinary sediment and in the renal biopsy. Under the polarized light microscope 2, 8 DHA crystals in urine appear as central Maltese cross appearance. Other ways to diagnose this are (iii) the measurement of levels of adenine in a 24-hour urine specimen with UV detection. (iv) stone analysis and (v) the molecular analysis of the APRT gene, as shown in Figure 3.
Algorithm for diagnosis of APRT deficiency
The stones are characteristically radiolucent and therefore, need to be differentiated from uric acid which is also radiolucent and have identical chemical reactivity. Infrared Spectrophotometry (IRS) or Fourier Transform Infrared microscopy (FTIR) provides characterization of the composition of crystals and confirms. Measurement of enzyme activity in red blood cell lysates is a useful tool for diagnosis of APRT deficiency.
Treatment; 2,8 DHA formation is blocked by Xanthine Oxidase blocker allopurinol or Fabuxostat. Adenine is then predominantly excreted in urine and it’s soluble in urine. Adult dose of Allopurinol is 400 mg / day ( Maximum 800mg/day) and in children 10 mg / kg/ day in absence of renal failure [13]. Fabuxostat, a recent XDH inhibitor may be used as an alternative therapy. Increased fluid intake is advisable. DHA crystal formation can be minimized by purine-poor diet to reduce adenine accumulation and by xanthine oxidase inhibition to prevent the alternative metabolism of adenine to DHA [3]. Urinary alkalization is not beneficial. Dialysis is ineffective in preventing DHA, as it is usually protein bound, and any removal on dialysis would be unable to keep up with the high rate of production [3]. Crystalluria can be used for monitoring treatment efficiency. The likelihood of complete regression of crystal deposition and recovery of allograft function depends on the extent of kidney damage at treatment initiation. In dialysis patients awaiting renal transplant it is preferable to initiate XDH inhibitors (Allopurinol or Fabuxostat) for better metabolic control prior to transplant. Medical management in APRT deficiency patients includes monitoring renal function, quantification of 2,8 DHA crystalluria and renal ultrasound every 6-12 monthly.
In summary, APRT deficiency causing 2, 8 DHA crystals is an under-recognized cause of irreversible renal failure with frequent recurrence in the allograft. Accurate early diagnosis and prompt pharmacologic inhibition of xanthine dehydrogenase may allow the stabilization or even lead to the improvement of graft function, reducing the risk of allograft loss.
- Sreejith P, Narasimhan KL, Sakhuja V (2009) 2, 8 Dihydroxyadenine urolithiasis: A case report and review of literature. Indian J Nephrol 19: 34-36. Link: http://bit.ly/35JZq2d
- Balasubramaniam GS, Arenas-Hernandez M, Escuredo E, Fairbanks L, Marinaki T, et al. (2016) Adenine phosphoribosyltransferase deficiency in the United Kingdom: two novel mutations and a cross-sectional survey. Clin Kidney J 9: 800-806. Link: http://bit.ly/3sorvpo
- Li J, Shingde M, Nankivell BJ, Tchan MC, Bose B, et al. (2019) Adenine Phosphoribosyltransferase Deficiency: A Potentially Reversible Cause of CKD. Kidney Int Rep 4: 1161-1170. Link: http://bit.ly/35HT1oh
- Fox IH, Meade JC, Kelley WN (1973) Adenine phosphoribosyltransferase deficiency in man. Report of a second family. Am J Med 55: 614-620. Link: http://bit.ly/3nOzgBo
- Daudon M (2014) Adenine Phosphoribosyltransferase Deficiency: An Under-Recognized Cause of Urolithiasis and Renal Failure. J Nephrol Ther 4: 4. Link: https://bit.ly/35HtDP8
- Edvardsson VO, Sahota A, Palsson R (2021) Adenine Phosphoribosyltransferase Deficiency. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJ, Mirzaa G, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle. Link: http://bit.ly/2XGOePl
- Parikh MD, Konnur A, Gang S (2019) Adenine phosphoribosyltransferase deficiency and 2, 8-dihydroxyadenine renal stones: A preventable cause of pediatric renal stones and kidney disease. Saudi J Kidney Dis Transplant 30: 723-725. Link: http://bit.ly/2XDYLeh
- Mockel L (2014) Adenine Phosphoribosyltransferase Deficiency: An Under-Recognized Cause of Urolithiasis and Renal Failure. J Nephrol Ther 04. Link: https://bit.ly/38M57OW
- Runolfsdottir HL, Palsson R, Agustsdottir IM, Indridason OS, Edvardsson VO (2016) Kidney Disease in Adenine Phosphoribosyltransferase Deficiency. Am J Kidney Dis 67: 431-438. Link: http://bit.ly/39ugjyS
- Runolfsdottir HL, Palsson R, Agustsdottir IM, Indridason OS, Edvardsson VO (2019) Long-term Renal Outcome of APRT Deficiency Presenting in Childhood. Pediatr Nephrol 34: 435-442. Link: http://bit.ly/35HTqah
- Zaidan M, Palsson R, Gall ECL, Garstka A, Maggiore U, et al. (2014) Recurrent 2,8-dihydroxyadenine nephropathy: a rare but preventable cause of renal allograft failure. Am J Transplant 14: 2623-2632. Link: http://bit.ly/3sqnTmM
- Bollee G, Daudon M, Ceballos-Picot I (2016) Adenine phosphoribosyltransferase deficiency: Leave no stone unturned. World J Clin Urol 3: 218-226. Link: https://bit.ly/39zYL4h
- Edvardsson VO, Runolfsdottir HL, Thorsteinsdottir UA, Agustsdottir IMS, Oddsdottir GS, et al. (2018) Comparison of the effect of allopurinol and febuxostat on urinary 2,8-dihydroxyadenine excretion in patients with APRT deficiency: a clinical trial. Eur J Intern Med 48: 75-79. Link: http://bit.ly/2XHNDgw
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
