Archives of Community Medicine and Public Health
Clinical features and outcomes of epidermolysis bullosa in thai children: A 20-Year review from a Tertiary Care Center
Ekamon Niltem*, Nootchanard Rujimethapass, Chonnakarn Sukhneewat, Wanida Limpongsanurak and Srisupalak Singalavanija
Cite this as
Niltem E, Rujimethapass N, Sukhneewat C, Limpongsanurak W, Singalavanija S (2022) Clinical features and outcomes of epidermolysis bullosa in thai children: A 20-Year review from a Tertiary Care Center. Arch Community Med Public Health 8(4): 140-146. DOI: 10.17352/2455-5479.000190Copyright License
© 2022 Niltem E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: Epidermolysis Bullosa (EB) is a heterogeneous genetic disorder with skin fragility. Only a few cases have been reported in Thailand. This study aims to determine the clinical characteristics, complications, and outcomes of EB stratified by subtype.
Methods: A retrospective single-center study of EB patients at the Dermatology Unit, Queen Sirikit National Institute of Child Health, was reviewed from January 1, 2002, to December 31, 2021. Diagnosis is based on clinical manifestations and some skin biopsies.
Results: There were 38 enrolled patients, age range from 0 to 25 years with a male-to-female ratio of 1.1:1. Family history of EB and consanguineous marriage were found in 6 cases and 2 cases, respectively. The most common type of EB was dystrophic EB (DEB) (26 cases) (68.4%), including recessive DEB in 15 cases (39.5%) and dominant DEB in 11 cases (28.9%). Other types were EB simplex in 10 cases (26.3%) and junctional EB in 2 cases (5.3%). Common complications were cutaneous bacterial infection (39.5%), anemia (31.6%), failure to thrive (18.4%), and protein energy malnutrition (15.8%). Musculoskeletal (21.1%), gastrointestinal (13.2%), and eye complications (7.9%) were exclusively found in DEB. Nineteen patients (50%) received regular follow-ups with a median duration of 9 months (range = 0.5 to 248 months). The mortality rate was 31.6% (6/19). Five cases died from bacterial sepsis, while one case died from metastatic squamous cell carcinoma.
Conclusion: DEB is the most common type of EB in Thai children, and bacterial sepsis is the predominant cause of death. Further multicenter and molecular genetic studies are recommended for a definite diagnosis.
Abbreviations
EB: Epidermolysis Bullosa; DEB: Dystrophic Epidermolysis Bullosa; EBS: Epidermolysis Bullosa Simplex; JEB: Junctional Epidermolysis Bullosa); KEB: Kindler Epidermolysis Bullosa; FTT: Failure to Thrive; SCC: Squamous Cell Carcinoma; DDEB: Dominant Dystrophic Epidermolysis Bullosa; RDEB: Recessive Dystrophic Epidermolysis Bullosa; AGA: Appropriate for Gestational Age; SGA: Small for Gestational Age; ASD: Atrial Septal Defect; VSD: Ventricular Septal Defect; GI: Gastrointestinal; NEBR: National Epidermolysis Bullosa Registry
Introduction
Epidermolysis bullosa (EB) is a prototype of genetic disorders with skin fragility [1-3] defined by skin blistering due to minimal mechanical trauma with disruption at the dermo-epidermal junctions, some of which are associated with significant morbidity and mortality. This disease occurs worldwide. It is estimated that the incidence varies in different countries between 1.4 – 41.3 per million live births [4-8]. According to the newest classification by consensus expert review in April 2019 [2], the four major classical EB types are EB simplex (EBS), skin cleavage within a basal layer of keratinocytes, junctional EB (JEB), skin cleavage through the lamina Lucida of the cutaneous basement membrane zone, dystrophic EB (DEB), skin cleavage at the sub-lamina data plane corresponding with the level of the anchoring fibrils and Kindler EB (KEB). EB is clinically and genetically heterogeneous, including a broad spectrum of severity with diversified subtypes. In severe cases, bullae and erosions can occur on any mucosal membrane and even be accompanied by extracutaneous involvement [1,2,9-12]. Complications and causes of death from EB, such as sepsis, pneumonia, respiratory failure, cardiomyopathy, undernutrition, failure to thrive (FTT), anemia, and squamous cell carcinoma (SCC) [1,9,13-19], have been elucidated extensively in the literature. Regardless, there are few case reports regarding the clinical features and outcomes of EB in Thailand [20-22].
The objectives of this study were to determine the clinical characteristics, complications, sequelae, and outcomes of EB patients in our hospital stratified by subtype.
Materials and methods
This retrospective study was conducted at the Dermatology Unit, Queen Sirikit National Institute of Child Health, the only Thai government hospital for children, in Bangkok, Thailand, over a 20-year period from January 1, 2002, to December 31, 2021. Eligibility for the inclusion of patients was based on the clinical features of blistering, peeling, erosions, or ulcerations from minimal mechanical trauma. All participants were systematically evaluated, diagnosed and, whenever possible, received skin biopsies to support the diagnosis and exclusion of another disease by the pediatric dermatologists at the institute. Classification of each type was primarily based on clinical signs and inheritance patterns, as described in Table 1.
Comprehensive data collection was identified from dermatology consultation notes, and inpatient and outpatient medical records. The demographic data, clinical features, extracutaneous manifestations, complications, sequelae, duration of follow-up, and outcomes were analyzed according to each subtype using the descriptive method with STATA statistical software (Version 14.0). This study was approved by the Research Ethic Review Committee of Queen Sirikit National Institute of Child Health (REC.058/2564).
Results
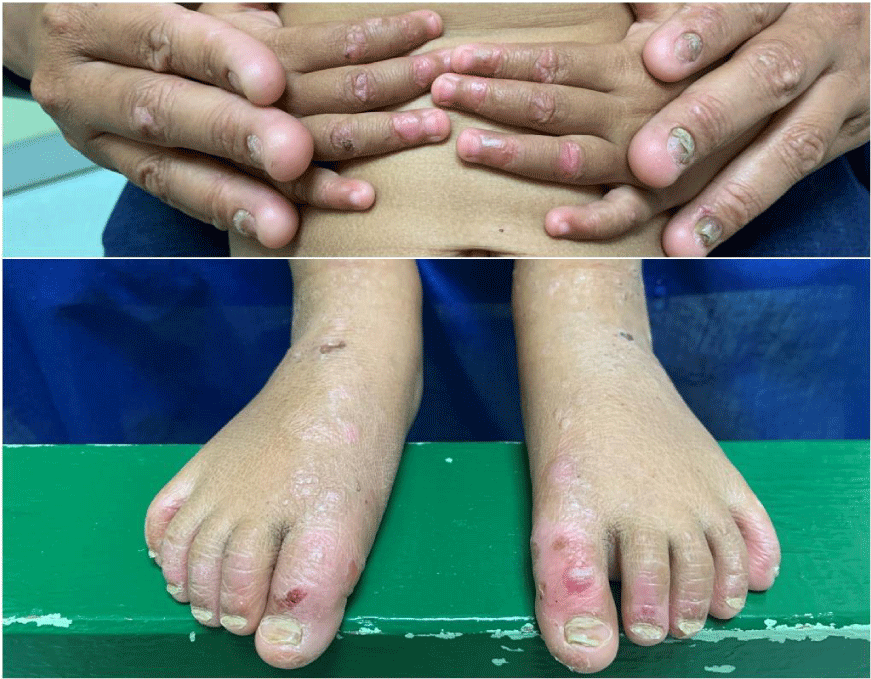
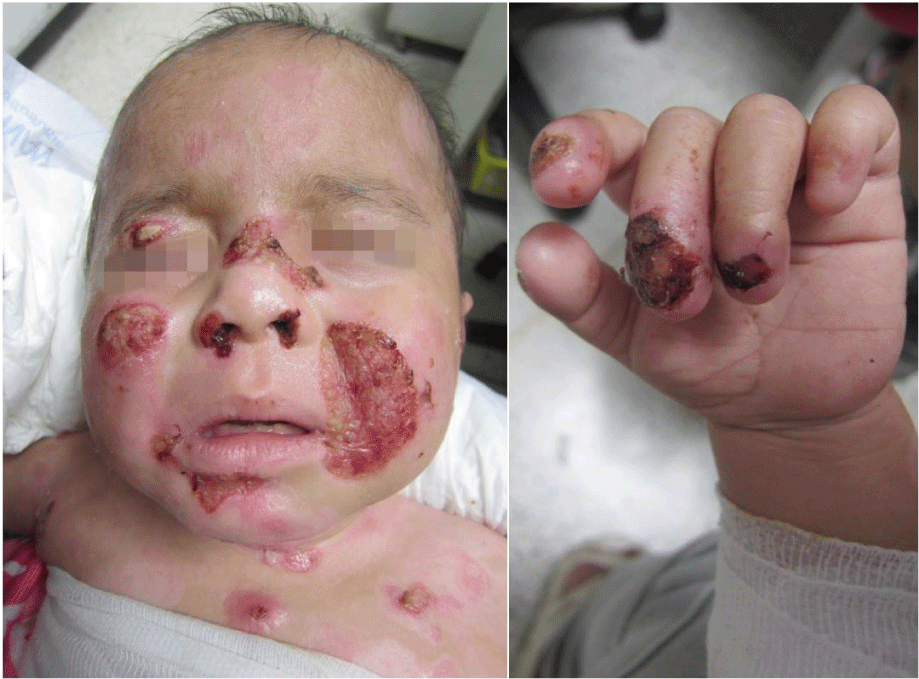
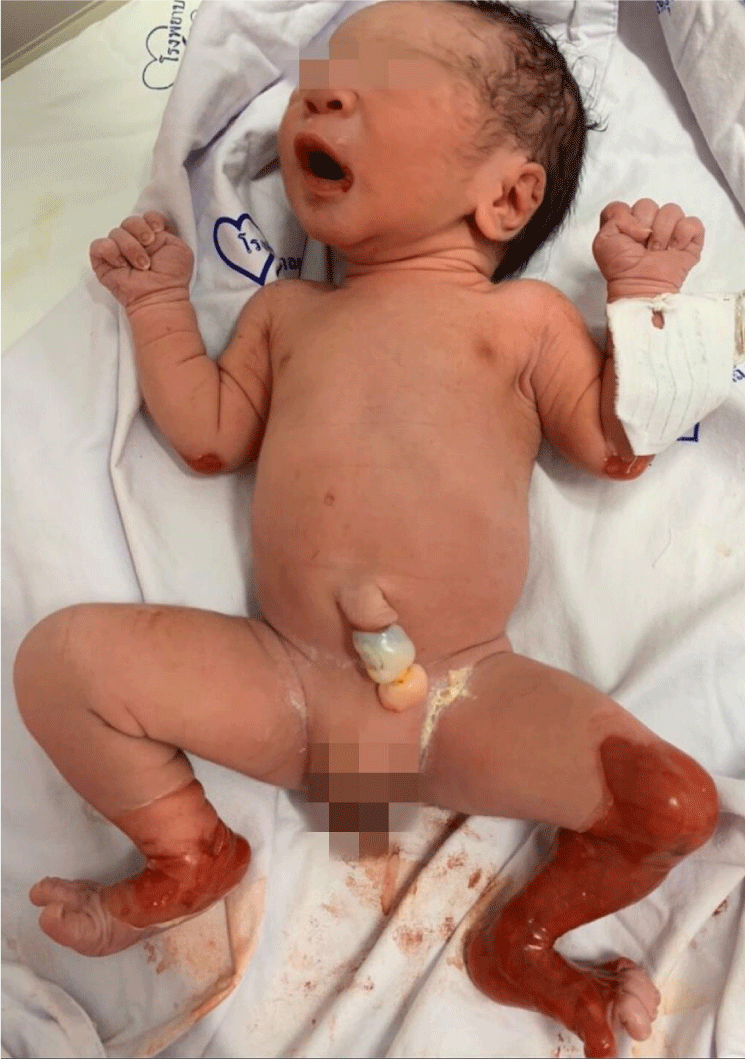
A total of 38 EB patients were enrolled, with an age range from 0 to 25 years. Skin biopsies were obtained from 14 patients (36.8%) to support the diagnosis of EB and rule out another vesiculobullous disease. The distribution of types of EB is summarized in Table 2. Based on typical clinical features, the most common type was DEB in 26 cases (68.4%) comprising dominant DEB (DDEB) (11/38, 28.9%) (Figure 1) and recessive DEB (RDEB) (15/38, 39.5%). Other major types were EBS in 10 cases (26.3%), localized EBS in 7 cases (7/38, 18.4%), intermediate EBS in 2 cases (2/38, 5.3%), and severe EBS (previously known as Dowling-Meara EBS) in 1 case (1/38, 2.6%). There were 2 cases of JEB (5.3%), 1 case of severe JEB (previously known as Herlitz JEB) (Figure 2), and 1 case of JEB with pyloric atresia. There was no Kindler EB. We also discovered EB with congenital absence of skin (Bart Syndrome) in 3 patients (Figure 3). All the Bart syndrome patients had typical cutaneous signs compatible with DEB. Table 3 shows the demographic data of all major EB categories. Twenty patients (52.6%) were male and 18 patients (47.4%) were female. The male-to-female ratio was 1.1:1. Family history of EB and consanguineous marriage were found in 6 cases (6/33, 18.2%) and 2 cases (2/32, 6.3%), respectively. Most of the patients had been born with no complications during labor. The deliveries were full-term (29/31, 93.5%) and appropriate for gestational age (AGA) (26/30, 86.7%). The geographical clustering of EB was crowded in Thailand’s central (26/38, 68.4%) and northeastern regions (8/38, 21.1%).
Clinical manifestations of EB (Table 4)
Cutaneous signs were present at birth in about half of the patients (20/38, 52.6%), meanwhile, the onset of clinical signs appeared in neonates (34.2%), infants (10.5%), and children (2.6%). As recorded when the diagnosis of EB was established, the frequency of predominant cutaneous lesions over various body parts was found in the extremities (20/38, 52.6%); head and neck (5/38, 13.2%); trunk and buttocks (2/38, 5.3%); and general areas (11/38, 28.9%). Extensive granulation tissue was demonstrated in one JEB patient, while milia were found in 12 patients (12/38, 31.6%), all of whom were classified as DEB (12/26, 46.2%). Oral mucosal involvement was found in 12 cases (31.6%). In addition, laryngeal mucosal involvement causing upper airway obstructive symptoms were found in 2 cases with severe EBS and severe JEB. Enamel hypoplasia was found in 2 cases (5.3%). Nail changes were detected in half of the patients (19/38, 50%), predominantly in DEB (16/26, 61.5%), JEB (1/2, 50%), and EBS (2/10, 20%), respectively. Anonychia was the most common nail sign (23.7%), followed by onychodystrophy (18.4%), and hyperkeratotic nails (7.9%). Alopecia was absent in all patients. Four patients (1 JEB patient and 3 DEB patients) had congenital anomalies; pyloric atresia; pannus formation of both eyes; ASD, VSD; and unilateral renal agenesis.
Sequelae and complications of EB (Table 5)
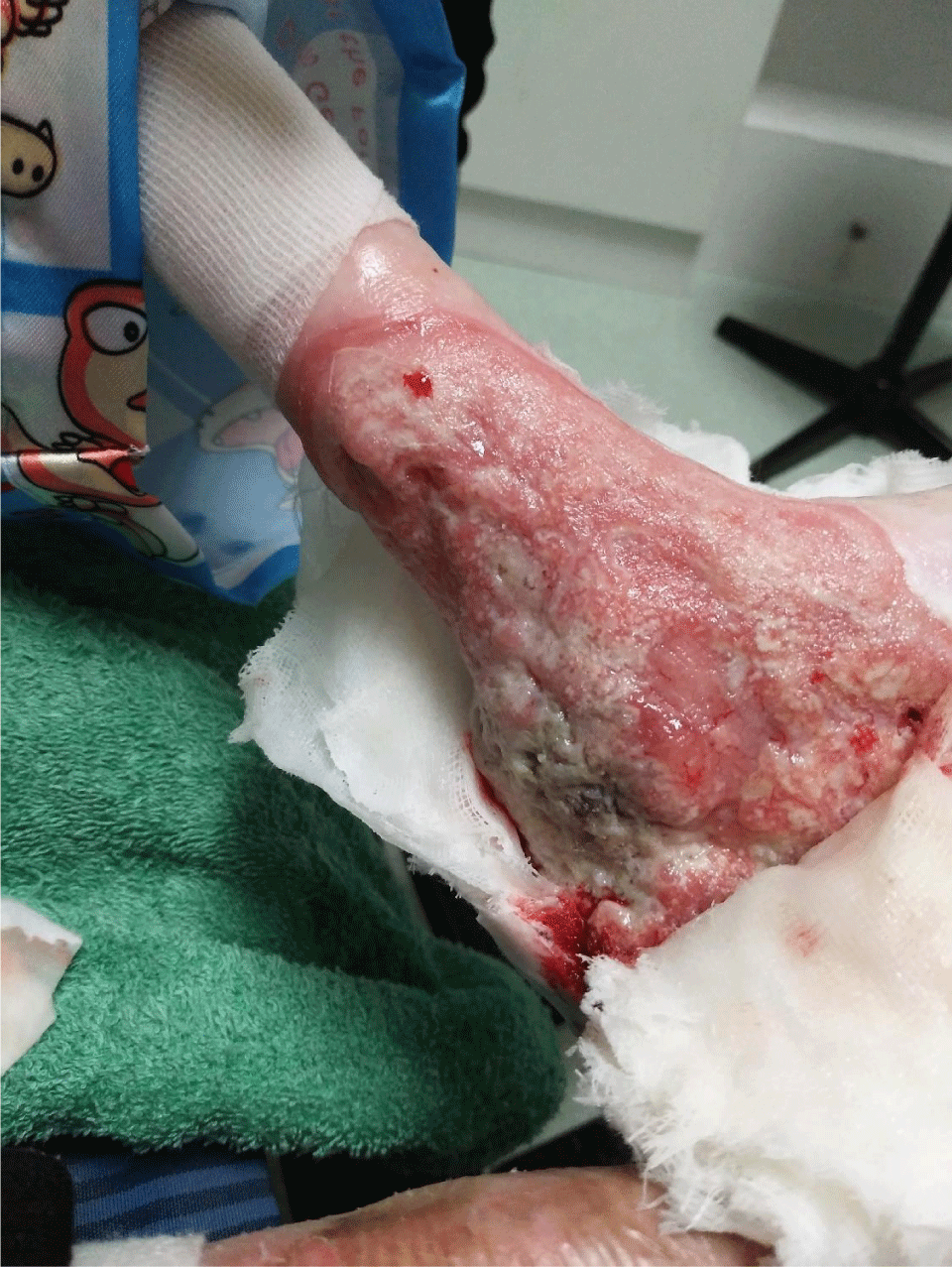
According to the types of EB, punctum stricture, xerophthalmia, and limbal stem cell defects, ocular complications were reported in 3 DEB patients (3/26, 11.5%). A variety of gastrointestinal (GI) involvements such as stenosis, stricture, constipation, and dysphagia were notable in 5 cases of DEB (19.2%). Cardiomyopathy and chronic renal failure were found in 2 cases of RDEB. All musculoskeletal deformities such as pseudo-syndactyly, scarring contracture, and mitten deformity were found in DEB (19.2%, 7.7%, and 3.9%, respectively). All neurodevelopmental abnormalities such as delayed milestones and intellectual disability occurred in 3 DEB patients (2/26, 7.7%, and 1/26, 3.9%). Not uncommonly, cutaneous bacterial infection was a complication in all types of EB (40% in EBS, 50% in JEB, and 34.6% in DEB). Pneumonia occurred in 4 cases (10.5%), mainly in the DEB type. Nutritional complications revealed iron deficiency anemia in 12 cases (31.6%), FTT in 7 cases (18.4%), and protein energy malnutrition (PEM) in 6 cases (15.8%). A majority of malnutrition was categorized in DEB. Unfortunately, 1 RDEB patient developed biopsy-proven well-differentiated cutaneous SCC over a chronic unhealing wound on his left ankle when he was 18 years old (Figure 4). He underwent below-knee amputation and chemotherapy.
Outcome of EB
The median duration of follow-up was 9 months (ranging from 0.5 to 248 months). Nineteen patients (50%) received regular follow-ups at our institute. The mortality rate was 31.6%, as 6 of the 19 cases proved fatal during the investigation period. The fatality rate was highest in JEB (2/2, 100%), compared to RDEB (3/9, 33.3%), and EBS (1/4, 25%). Five cases (83.3%) comprising a single case of intermediate EBS, 2 JEB- and 2 of RDEB-patients died from uncontrolled blood culture-proven bacterial sepsis with disseminated intravascular coagulation, while one case (16.7%) diagnosed with RDEB was fatal due to metastatic SCC in the inguinal lymph nodes when he was 20 years old. Amongst the fatalities, the median life span was 6 months (ranging from 2 to 245 months). There were at least 13 survivors, which revealed good clinical recovery (61.5%; 8/13), stable clinical courses (30.8%; 4/13), and progressively deteriorated outcomes (7.7%; 1/13), who was diagnosed with RDEB and developed dilated cardiomyopathy.
Discussion
This first retrospective study of EB in Thailand over a 20-year period was designed to determine the epidemiology, clinical characteristics, spectrum of extracutaneous features, diversity of complications, and outcomes of this condition stratified by specific type. Based on our institute data, the most frequently diagnosed type was DEB (68.4%), which is consistent with most studies from tertiary care centers [14,17,23-25], where DEB was identified as the most frequently discovered type (59.8-77.2%). Notably, the proportion of EBS patients in this study (26.3%) was relatively lower, whereas the DEB percentage was relatively higher than the reports of the National Epidermolysis Bullosa Registry (NEBR) on information from the Netherlands, United States, Australia, New Zealand, England, and Wales [4,6-8]. According to research from the NEBR database [4,6-8], the outstanding type was EBS, which accounted for 45.7-53.7%. This was followed by DEB (34.7-35.2%). A comparison of the distribution of EB types in this work with previous studies conducted in other regions of the world during the last decade is summarized in Table 6; the chart also emphasizes differences in study periods, database sources, patient numbers, and additional diagnostic methods apart from clinical assessment. Several reasons could be explanations for these findings.
First, localized EBS is the most common clinical variant among EBS main subtypes. Although skin blistering develops in early infancy, it may not appear until early adulthood and is usually confined to the hands and feet. Lesions often heal without scarring and no obvious extracutaneous involvement [1,2], which might be misdiagnosed by a primary physician or pediatrician. Furthermore, the natural history of mild symptoms and tendency to blister gradually disappear in adolescence [1], which might be underestimated or neglectable in referring to tertiary care centers. Conversely, more DEB patients tend to be referred to tertiary hospitals than localized EBS patients due to greater severity with functional incapacity from extensive scarring [13,14,24]. We believe that the calculated EBS percentage will increase when the NEBR is fully set up in Thailand and truly reflects the nationwide statistics. Second, the diagnostic gaps of different EB types in low-outsource countries are problematic, which may affect the reliability of diagnosis. In the past, genetic testing or even immunofluorescence mapping was not feasible in all institutes. Nonetheless, modern methods for genetic testing in EB include next-generation sequencing and whole-exome sequencing, both of which are increasingly used in Thailand. Finally, global variations in populations, ethnicity, and inbreeding culture in some regions may affect the distribution of EB subtypes [26,27].
EB is also associated with many distinctive extracutaneous manifestations as well as various sequelae contributing to the disease burden. We found that clinical pictures such as external eyes (punctum stricture, limbal stem cell defect, xerophthalmia), dilated cardiomyopathy, gastrointestinal complications (dysphagia, constipation, structuring), chronic kidney disease, musculoskeletal deformities, iron deficiency anemia and failure to thrive appear in DEB, and RDEB specifically, more than other subtypes, which is consistent with a number of previous studies [2,9-14].
The fatality rate of EB from our institute was 31.6% and the highest in JEB (100%), comparable with a previous 11-year retrospective study from two centers in South Korea where Kim, et al. reported that five mortalities were caused by sepsis, failure to thrive, and severe metabolic acidosis with dehydration from a total of 30 patients; the highest mortality rate was noted in patients with JEB (up to 75%) [17]. As a matter of fact, bacterial sepsis is the most common cause of death during infancy in all subtypes of EB. Similarly, several studies supported sepsis as a major cause of early death in this work [11,15-17]. In addition, malabsorption with secondary failure to thrive, severe metabolic acidosis, tracheolaryngeal complications, or respiratory failure were other prominent causes of death [15-17].
Despite aggressive surgical resection in metastatic SCC, cardiomyopathy and chronic renal failure are the main causes of death in patients with RDEB who survive beyond childhood. The cumulative risks of SCC by age 20 start at 7.5%, then dramatically increase to 90.1% by age 55 with the median survival from the first diagnosis at only 2.4 years [18,19]. Our RDEB patient who developed metastatic SCC also had severe iron deficiency anemia, despite regular red-cell transfusions due to chronic blood loss from his wounds. Furthermore, he suffered from dysphagia because esophageal stricture led to insufficient intake of protein, energy, and nutrients. We highlight that comprehensive multidisciplinary care, despite no currently approved curative therapies, comprising protection from friction, avoidance of abrasion, control of secondary infection, pain and itch relief, nutritional supplementation, awareness of growth failure, long-term surveillance for SCC (starting in later adolescence), and psychosocial palliative will provide better outcomes for affected individuals and families, particularly in life-long, distressing RDEB.
This study had some limitations, including its single-center retrospective design. In Thailand, an official nationwide registry of EB has not yet been established. Consequently, we do not have data on all the known cases of EB in Thailand, and the findings may not reflect the overall data or be generalized to other healthcare settings. However, most EB in pediatric patients is diagnosed and treated at our center. It is important to note that nearly all patients were diagnosed and categorized by expert pediatric dermatologists into various types or subtypes of EB on the basis of clinical information, inheritance patterns, histopathology, and/or transmission electron microscopic study (in only one case), even though clinical identification of the major types of EB is unreliable exclusively in neonates [2,3]. Consequently, molecular genetic diagnosis should be encouraged to determine accurate subtypes, enable prompt genetic counseling, and improve prognostication [2,28].
Conclusion
DEB is the most common classical type of EB in Thai children. A wide range of extracutaneous manifestations and complications were seen in each subtype. Not uncommonly, bacterial sepsis was the predominant cause of death. Our report on this study of EB provides fundamental information that will contribute to use as a reference for clinical courses and prognosis in Thai patients with the eventual development of plans for future comprehensive management to improve quality of life and refine outcomes for patients with EB. Further multicenter and molecular genetic studies are recommended to confirm the findings of this study.
- Paller SA, Mancini JA. Bullous Disorders of Childhood. In: Paller and Mancini – Hurwitz Clinical Pediatric Dermatology. 6th ed. U.S.A.: Elsevier; 2022; 369-390.
- Has C, Bauer JW, Bodemer C, Bolling MC, Bruckner-Tuderman L, Diem A, Fine JD, Heagerty A, Hovnanian A, Marinkovich MP, Martinez AE, McGrath JA, Moss C, Murrell DF, Palisson F, Schwieger-Briel A, Sprecher E, Tamai K, Uitto J, Woodley DT, Zambruno G, Mellerio JE. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020 Oct;183(4):614-627. doi: 10.1111/bjd.18921. Epub 2020 Mar 11. PMID: 32017015.
- Lucky AW, Whalen J, Rowe S, Marathe KS, Gorell E. Diagnosis and Care of the Newborn with Epidermolysis Bullosa. Neoreviews. 2021 Jul;22(7):e438-e451. doi: 10.1542/neo.22-7-e438. Epub 2021 Jul 1. PMID: 34210808.
- Baardman R, Yenamandra VK, Duipmans JC, Pasmooij AMG, Jonkman MF, van den Akker PC, Bolling MC. Novel insights into the epidemiology of epidermolysis bullosa (EB) from the Dutch EB Registry: EB more common than previously assumed? J Eur Acad Dermatol Venereol. 2021 Apr;35(4):995-1006. doi: 10.1111/jdv.17012. Epub 2020 Nov 16. PMID: 33095945; PMCID: PMC7984089.
- Dănescu S, Has C, Senila S, Ungureanu L, Cosgarea R. Epidemiology of inherited epidermolysis bullosa in Romania and genotype-phenotype correlations in patients with dystrophic epidermolysis bullosa. J Eur Acad Dermatol Venereol. 2015 May;29(5):899-903. doi: 10.1111/jdv.12709. Epub 2014 Sep 8. PMID: 25201089.
- Fine JD. Epidemiology of Inherited Epidermolysis Bullosa Based on Incidence and Prevalence Estimates From the National Epidermolysis Bullosa Registry. JAMA Dermatol. 2016 Nov 1;152(11):1231-1238. doi: 10.1001/jamadermatol.2016.2473. PMID: 27463098.
- Petrof G, Papanikolaou M, Martinez AE, Mellerio JE, McGrath JA, Bardhan A, Harper N, Heagerty A, Ogboli M, Chiswell C, Moss C. The epidemiology of epidermolysis bullosa in England and Wales: data from the national epidermolysis bullosa database. Br J Dermatol. 2022 May;186(5):843-848. doi: 10.1111/bjd.20958. Epub 2022 Mar 31. PMID: 34927719.
- Kho YC, Rhodes LM, Robertson SJ, Su J, Varigos G, Robertson I, Hogan P, Orchard D, Murrell DF. Epidemiology of epidermolysis bullosa in the antipodes: the Australasian Epidermolysis Bullosa Registry with a focus on Herlitz junctional epidermolysis bullosa. Arch Dermatol. 2010 Jun;146(6):635-40. doi: 10.1001/archdermatol.2010.109. PMID: 20566927.
- Fine JD, Bruckner-Tuderman L, Eady RA, Bauer EA, Bauer JW, Has C, Heagerty A, Hintner H, Hovnanian A, Jonkman MF, Leigh I, Marinkovich MP, Martinez AE, McGrath JA, Mellerio JE, Moss C, Murrell DF, Shimizu H, Uitto J, Woodley D, Zambruno G. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014 Jun;70(6):1103-26. doi: 10.1016/j.jaad.2014.01.903. Epub 2014 Mar 29. PMID: 24690439.
- Fine JD, Mellerio JE. Extracutaneous manifestations and complications of inherited epidermolysis bullosa: part I. Epithelial associated tissues. J Am Acad Dermatol. 2009 Sep;61(3):367-84; quiz 385-6. doi: 10.1016/j.jaad.2009.03.052. PMID: 19700010.
- Fine JD, Mellerio JE. Extracutaneous manifestations and complications of inherited epidermolysis bullosa: part II. Other organs. J Am Acad Dermatol. 2009 Sep;61(3):387-402; quiz 403-4. doi: 10.1016/j.jaad.2009.03.053. PMID: 19700011.
- Laimer M, Prodinger C, Bauer JW. Hereditary epidermolysis bullosa. J Dtsch Dermatol Ges. 2015 Nov;13(11):1125-33. doi: 10.1111/ddg.12774. PMID: 26513070.
- Tang JY, Marinkovich MP, Lucas E, Gorell E, Chiou A, Lu Y, Gillon J, Patel D, Rudin D. A systematic literature review of the disease burden in patients with recessive dystrophic epidermolysis bullosa. Orphanet J Rare Dis. 2021 Apr 13;16(1):175. doi: 10.1186/s13023-021-01811-7. PMID: 33849616; PMCID: PMC8045359.
- Feinstein JA, Jambal P, Peoples K, Lucky AW, Khuu P, Tang JY, Lara-Corrales I, Pope E, Wiss K, Hook KP, Levin LE, Morel KD, Paller AS, McCuaig CC, Powell J, Eichenfield LF, Price H, Levy ML, Schachner LA, Browning JC, Bayliss S, Jahnke M, Shwayder T, Glick SA, Bruckner AL. Assessment of the Timing of Milestone Clinical Events in Patients With Epidermolysis Bullosa From North America. JAMA Dermatol. 2019 Feb 1;155(2):196-203. doi: 10.1001/jamadermatol.2018.4673. PMID: 30586139; PMCID: PMC6439540.
- Fine JD, Johnson LB, Weiner M, Suchindran C. Cause-specific risks of childhood death in inherited epidermolysis bullosa. J Pediatr. 2008 Feb;152(2):276-80. doi: 10.1016/j.jpeds.2007.06.039. Epub 2007 Oct 22. PMID: 18206702.
- Hon KL, Li JJ, Cheng BL, Luk DC, Murrell DF, Choi PC, Leung AK. Age and etiology of childhood epidermolysis bullosa mortality. J Dermatolog Treat. 2015 Apr;26(2):178-82. doi: 10.3109/09546634.2014.915002. Epub 2014 May 15. PMID: 24724596.
- Kim KY, Namgung R, Lee SM, Kim SC, Eun HS, Park MS, Park KI, Lee C. Nutritional outcomes in children with epidermolysis bullosa: the experiences of two centers in Korea. Yonsei Med J. 2014 Jan;55(1):264-9. doi: 10.3349/ymj.2014.55.1.264. PMID: 24339316; PMCID: PMC3874902.
- Fine JD, Johnson LB, Weiner M, Li KP, Suchindran C. Epidermolysis bullosa and the risk of life-threatening cancers: the National EB Registry experience, 1986-2006. J Am Acad Dermatol. 2009 Feb;60(2):203-11. doi: 10.1016/j.jaad.2008.09.035. Epub 2008 Nov 20. PMID: 19026465.
- Robertson SJ, Orrin E, Lakhan MK, O'Sullivan G, Felton J, Robson A, Greenblatt DT, Bernardis C, McGrath JA, Martinez AE, Mellerio JE. Cutaneous Squamous Cell Carcinoma in Epidermolysis Bullosa: a 28-year Retrospective Study. Acta Derm Venereol. 2021 Aug 24;101(8):adv00523. doi: 10.2340/00015555-3875. PMID: 34230977; PMCID: PMC9413672.
- Singalavanija S, Phuvichit B, Palungwachira P. Epidermolysis bullosa letalis (Herlitz disease): a case report. J Med Assoc Thai. 1994 Feb;77(2):103-7. PMID: 7798834.
- Suwanthaweemeesuk J, Phunmanee C, Singthong S, Kwangsukstid O, Supsrisunjai C. Epidermolysis Bullosa Pruriginosa: A Report of Siblings. Int J Dermatol Venereol. 2022.
- Pongmee P, Wittayakornrerk S, Lekwuttikarn R, Pakdeeto S, Watcharakuldilok P, Prempunpong C, Tim-Aroon T, Puttanapitak C, Wattanasoontornsakul P, Junhasavasdikul T, Wongkittichote P, Noojarern S, Wattanasirichaigoon D. Epidermolysis Bullosa With Congenital Absence of Skin: Congenital Corneal Cloudiness and Esophagogastric Obstruction Including Extended Genotypic Spectrum of PLEC, LAMC2, ITGB4 and COL7A1. Front Genet. 2022 Apr 1;13:847150. doi: 10.3389/fgene.2022.847150. PMID: 35432467; PMCID: PMC9010945.
- Yu Y, Wang Z, Mi Z, Sun L, Fu X, Yu G, Pang Z, Liu H, Zhang F. Epidermolysis Bullosa in Chinese Patients: Genetic Analysis and Mutation Landscape in 57 Pedigrees and Sporadic Cases. Acta Derm Venereol. 2021 Jul 15;101(7):adv00503. doi: 10.2340/00015555-3843. PMID: 34046686; PMCID: PMC9413781.
- Farokhforghani S, Fatemi MJ, Ghanooni P, Asadpour F, Araghi S, Nouri A. Epidermolysis Bullosa Registry Data in Iran. World J Plast Surg. 2021 Sep;10(3):99-103. doi: 10.29252/wjps.10.3.99. PMID: 34912673; PMCID: PMC8662693.
- Nanda A, Liu L, Al-Ajmi H, Al-Saleh QA, Al-Fadhli S, Anim JT, Ozoemena L, Mellerio JE, McGrath JA. Clinical subtypes and molecular basis of epidermolysis bullosa in Kuwait. Int J Dermatol. 2018 Sep;57(9):1058-1067. doi: 10.1111/ijd.14099. Epub 2018 Jul 16. PMID: 30011071.
- Abu Sa'd J, Indelman M, Pfendner E, Falik-Zaccai TC, Mizrachi-Koren M, Shalev S, Ben Amitai D, Raas-Rothshild A, Adir-Shani A, Borochowitz ZU, Gershoni-Baruch R, Khayat M, Landau D, Richard G, Bergman R, Uitto J, Kanaan M, Sprecher E. Molecular epidemiology of hereditary epidermolysis bullosa in a Middle Eastern population. J Invest Dermatol. 2006 Apr;126(4):777-81. doi: 10.1038/sj.jid.5700163. Erratum in: J Invest Dermatol. 2006 Jun;126(6):1427. PMID: 16439963.
- Murata T, Masunaga T, Ishiko A, Shimizu H, Nishikawa T. Differences in recurrent COL7A1 mutations in dystrophic epidermolysis bullosa: ethnic-specific and worldwide recurrent mutations. Arch Dermatol Res. 2004 Mar;295(10):442-7. doi: 10.1007/s00403-003-0444-1. Epub 2004 Jan 16. PMID: 14727126.
- Has C, Liu L, Bolling MC, Charlesworth AV, El Hachem M, Escámez MJ, Fuentes I, Büchel S, Hiremagalore R, Pohla-Gubo G, van den Akker PC, Wertheim-Tysarowska K, Zambruno G. Clinical practice guidelines for laboratory diagnosis of epidermolysis bullosa. Br J Dermatol. 2020 Mar;182(3):574-592. doi: 10.1111/bjd.18128. Epub 2019 Aug 9. PMID: 31090061; PMCID: PMC7064925.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
