Archives of Community Medicine and Public Health
Studies on prevalence of malaria and its adverse fetal outcomes in Federal Medical Centre (FMC), Owerri, IMO State, Nigeria
Iwuchukwu IC* and Vincent CN
Cite this as
Iwuchukwu IC, Vincent CN (2021) Studies on prevalence of malaria and its adverse fetal outcomes in Federal Medical Centre (FMC), Owerri, IMO State, Nigeria. Arch Community Med Public Health 7(2): 151-163. DOI: 10.17352/2455-5479.000156Despite a massive increase in private and public efforts over the last years, malaria remains one of the most salient global health concerns. The study adopted cross sectional and descriptive survey design to assess the prevalence of maternal malaria and its adverse fetal outcomes in Federal Medical Centre (FMC), Owerri, Imo State, Nigeria from September, 2020 to March, 2021. The study population were 814 consented pregnant women in their reproductive ages (16 - 55years) who attended ante natal clinic or delivered of their babies at FMCO during the time of study. Data collection involved administration of closed ended questionnaire to illicit information on biographic data. Clinical assessments/examinations (laboratory investigations) of maternal peripheral blood, and fetal birth weight were utilized. Shortly before child birth maternal peripheral blood was obtained from each participant into sterile container for laboratory analysis. Statistical analysis of generated data was carried out using descriptive analysis and of percentages and presented using tables. Statistical comparisons and test of significance between positive and negative groups were calculated using the non-parametric Chi-square test. Differences were considered significant at P< 0.05. The study revealed that 65.6% had malaria during pregnancy. Malaria prevalence is significantly associated with maternal age bracket ( x2= 16.27; P < 0.05), gravidity (x2 = 14.9; P < 0.05) and level of education (x2= 24.69; P < 0.05). There is significant relationship between maternal malaria and perinatal mortality (x2 = 23.14; P < 0.05). There is significant effect of maternal malaria on perinatal mortality based on maternal age (x2= 40.11; P < 0.05) and gravidity (x2= 48.67; P < 0.05). An overall prevalence of preterm deliveries were 19.7%. There is significant relationship between maternal malaria and preterm delivery (x2 = 27.58; P < 0.05). There is significant effect of maternal malaria on preterm delivery based on maternal age (x2 = 49.2; P < 0.05) and gravidity (x2= 56.94; P < 0.05). An overall prevalence of low birth weight were 23.6%. There is significant relationship between maternal malaria and fetal birth weight (x2 = 34.06; P < 0.05). There is significant effect of maternal malaria on fetal birth weight based on maternal age (x2 = 53.82; P < 0.05) and gravidity (x2= 65.94; P < 0.05). The study suggests effective therapy since perinatal mortality due to maternal malaria was recorded in this study. Preterm deliveries and low fetal birth weight based on gravidity and maternal age groups associated with maternal malaria as identified is a call for program managers to make haste and implement new strategies for malaria control.
Introduction
In malaria endemic areas, pregnant women are the highest risk group for malaria infection and to develop a severe form of the disease that results in mortality. Thus, increasing the use of antimalaria interventions that target pregnant women which can address the social, cultural, and economic factors that heighten susceptibility has the potential to control the disease in most of the susceptible and underserved groups [1].
Infection of malaria during pregnancy is common, which can result in fetus low birth weight, stillbirth, and decrement in intrauterine fetal growth. Besides, malaria infection has the greatest impact on the survival of mothers. The factor behind the high burden of malaria during pregnancy could be the increased body surface and specific odor secretions during pregnancy which may expose them to increased mosquito bites [1,2].
Malaria is one of the killer diseases worldwide. According to the World Health Organization (WHO) report in 2016, around 216 million new cases of malaria occurred globally. Besides, most of the malaria cases were in the African region (90%) followed by the Southeast Asia region (7%) and Eastern Mediterranean region (2%). Similarly, there was an estimated 445000 malaria deaths worldwide. Most of these deaths occurred in the Africa region (91%) followed by the Southeast Asia region (6%) and Eastern Mediterranean region (2%) [3].
Malaria infection during pregnancy is a major public health concern in tropical and subtropical countries with significant risk for the pregnant woman and her fetus. According to the estimated yearly report, the number of pregnant women who were at risk of malaria was about 25 million [4]. The rate of malaria infection is higher in pregnant women because of their decreased immunity. Mainly pregnant women living in areas of low or unstable malaria transmission have little or no immunity to malaria and are at higher risk of developing the severe disease as a result of malaria infection than non-pregnant adults living in the same area. Pregnant women with malaria have an increased risk of abortion, stillbirth, premature delivery, and low-birthweight infants [5,6]. Moreover, in unstable malaria transmission areas, pregnant mothers’ death may be due to complications of severe malaria (hypoglycaemia, cerebral malaria, and pulmonary edema) or indirectly from malaria-related severe anemia [5].
In order to prevent malaria in pregnancy, current WHO guidelines recommend a multi-pronged approach including both preventive and curative measures [5]. The Focused Antenatal Care approach recommends the use of Insecticide-Treated Nets (ITN) early in pregnancy and a minimum of two treatment doses of Sulphadoxine-Pyrimethamine (SP) as Intermittent Preventive Treatment in Pregnancy (IPTp) (WHO, 2008). Despite these recommendations, effective access to malaria prevention in pregnancy remains limited in Nigeria. According to Nigeria Malaria Fact Sheet (2011), only 11.8% of pregnant women slept under an ITN, and only 6.5% of pregnant women had taken the recommended two doses of SP during pregnancy [7]. Accordingly, the prevalence of malaria in pregnancy remains high, with recent estimates suggesting prevalence rates of close to 50% in the second and third trimesters. Plasmodium falciparum infections of the placenta remain a major medical challenge among pregnant women in sub-Saharan Africa. A number of factors influence the prevalence of placental malaria in pregnant women, including maternal age, gravidity, use of prophylaxis, nutrition, host genetics, and level of antiparasite immunity, as well as parasite genetics and transmission rates [8].
Twelve years after the first Abuja declaration, Nigeria failed to halve the malaria burden in 2010. In the next 2 years leading up to the Millennium Development Goals’ (MDG) deadline, Nigeria is still recording high prevalence (98.4%) of malaria (Ako-Nai and Adesiyan, 2012), hence it is doubtful if Nigeria could halt malaria by 2020 and begin to reverse the incidence. The failure to consider community’s knowledge, attitudes and practices (KAP) about malaria has contributed to the inability of programs to achieve sustainable control (Tyagi, Roy and Malhotra, 2013). People’s behavior may increase malaria risk, but to change such behavior is not easy. Indeed, there are many reasons why particular behaviors exist and they often are tied to considerable benefits in areas quite distinct from health. Thus, it is not usually the case that “these people don’t know any better”, but rather that their native logic and rationality make sense within the realities and limitations of their local circumstances [9].
Despite a massive increase in private and public efforts over the last years, malaria remains one of the most salient global health concerns. Approximately one in four women show evidence of placental infection at the time of delivery, with a large fraction of infection remaining undetected and untreated [10]. The health consequences of malaria infection during pregnancy are large: malaria-induced low birthweight is estimated to account for up to 360,000 infant deaths every year [11]; overall, 11.4% of neonatal deaths and 5.7% of infant deaths in malaria-endemic areas of Africa are estimated to be caused by malaria in pregnancy [12,13].
Recent data from Imo State indicate that total loss due to malaria in pregnancy within a six month period was estimated at 5.8 million naira, suggesting that the burden of malaria in pregnant women is high [14]. It has also been suggested that anaemia might be associated with low birth weight [15]. However low birth weight might be due to malaria-induced pathological lesions that occur in the placenta leading to intrauterine growth retardation [16]. This may occur following placental malaria parasitisation which is common in Plasmodium falciparum infection in pregnant women who live in malaria endemic countries [15]. Placental malaria poses a great challenge in malaria control strategies in that it may occur in asymptomatic parasitaemic as well as aparasitaemic pregnant women [17]. Studies have shown that parasitized erythrocytes tend to sequester in the placental capillaries leading to hypoxia, inflammatory reactions and chronic intervellitis [18]. Therefore, while the placenta of infected pregnant women may be full of parasitised erythrocytes, with parasite densities sometimes in excess of 50% of the total placental erythrocyte count, the peripheral blood may remain free of parasites [19]. Consequently, interventions directed towards symptomatic parasitaemic pregnant women may leave out those who are equally at risk of anaemia and low birth weight. Information on epidemiology and socio-economic consequences of malaria in pregnant women in Imo State has been documented [12].
However, there are no recent studies and therefore this study was set to assess the prevalence of malaria and its adverse fetal outcomes in Federal Medical Centre (FMC), Owerri, Imo State, Nigeria. The publication of this research will alert the pregnant women, community, voluntary and government agencies on the cases of malaria during pregnancy with its resultant effect on the fetus and the infant. The result of this research will arouse the interest of the government and voluntary agencies (WHO) towards providing necessary materials, manpower, infrastructure needed to combat malaria in our society.The data collected on this research will serve to awaken doctors, nurses, environmental health workers and other health workers to great danger posed by malaria to the public thereby motivating them towards sensitizing the general public on preventive/control measures of malaria. Furthermore, the result of this research would motivate environmental health workers for further research on how to combat mosquito in the environment so as to reduce its transmission of Plasmodium to individuals and the general populace. Perhaps solution of malaria controls lies -in primary care physicians such as family physician or community health workers working in the rural communities. The result of this study will increase their current knowledge for health education and promotion on malaria at the first contact either in the health facilities or in the patient’s family house upon home visit. The findings of this research would also motivate the pregnant women to adopt positive attitude and good practice to control malaria during pregnancy so as to reduce its effect on their unborn babies. It will equally help them to understand causes, signs and symptoms, transmission and consequences of malaria during pregnancy which in turn would help them adopt preventive/control measures of malaria. The study would serve as a reference point to future research on malaria and will also help to add to the required literature on malaria in pregnancy. The findings of this research will go a long way in motivating curriculum planners towards enforcing the teaching of effects of malaria on the unborn child and also control measures to combat malaria during pregnancy in various levels of educational institution. Finally, the result of the study would motivate government and non-governmental agencies towards organizing conferences, seminars and workshops on roll back malaria strategies so as to benefit the masses.
This study is limited to the assessment of the effects of malaria in pregnancy among pregnant women in Federal Medical Centre, Owerri (FMCO), Imo State, Nigeria. It is also limited to modifying variables like, knowledge, attitudes and practices of pregnant women in FMCO. The study is further delimited to independent variables of gravidity and maternal age groups of the pregnant women who visited the hospital to receive ante natal care or delivered their babies and, also gave their consent to participate in the study. The study was limited to the period the study took place: September 202 to March 2021. The study would be useful to mothers especially the pregnant ones, nurses, doctors, environmental health personnel, health educators/counselors, curriculum planners, hospital management, and health workers as well as those concerned with the prevention and control of malaria like World Health Organization (WHO).
Research methodology
Research design: The study adopted cross sectional and descriptive survey design. Eligible women were approached for recruitment and those who consented were followed from their ante natal visit till they delivered of their babies. Stratified sampling technique was used together with simple random sampling. The women were grouped into weeks of antenatal visits and each week simple random sampling was used for those that came for antenatal visit to ensure equal chance of participation. Each morning, consenting women who met the study criteria was assigned numbers serially as they reported at the antenatal clinic. A client was picked at random from the first three eligible attendants. Every third eligible attendant from the one picked was given questionnaire to fill. Any woman who had filled questionnaire had her antenatal folder/card marked ‘BIO’ to avoid repeat recruitment during any subsequent clinic attendance. The copies of the questionnaire were administered to them before they receive health counseling.
Study area
The study was carried out at Obstetrics unit (ANC, Labour ward) of Federal Medical Centre, Owerri, Imo State, Nigeria, from September, 2020 to March, 2021. Federal Medical Centre Owerri (FMCO) lies in the south east of Nigeria. It is a federal government owned tertiary hospital situated in Owerri municipal. Owerri is the capital of Imo State in Nigeria. FMCO is located along Orlu road. It lies on a land perimeter of 359 kilometers. At maximum, the hospital can be transformed to accommodate between 500 – 850 inpatients at one time. It has several wards, three laboratories, two radiology laboratories and one support services and departments. FMCO is an apex health institution where complicated medical conditions are managed or treated. It also functions as a training ground for intern physicians, physiotherapists, pharmacists, and nurses who are trained on the job. It ideally offers specialized health care for inpatients and outpatients on referral from primary and secondary facilities. Its offers services related to cancer management, neurosurgery, cardiac surgery, plastic surgery, burns repair, palliative care, advanced obstetrics, neonatology, gynaecology and paediatric services. It serves an teaming population of about 127,213 persons in Owerri municipal alone, and about 1,407,000 persons from outside Owerri municipal (FMCO Records Unit, 2019). The hospital has a total staff strength of 1,674. FMCO takes an estimated 280 births per a month. This makes FMCO a suitable place to carry out this study on prevalence of malaria and its adverse effects on the woman and the fetus.
Population
The study population were consented pregnant women in their reproductive ages (16 - 55years) who attended ante natal clinic or delivered of their babies at FMCO during the time of study; September 2020 to March 2021. This was made up of two thousand nine hundred and forty six (2,946) women. The study subjects for specimen collection and laboratory studies were nine hundred and eighty two (982) women. Of these, 168 (representing 17.1%) were not included in the analysis: 96 subjects were lost from maternal death and change of location/hospital; 23 slides were not read because they were not properly prepared; while 49 women lost interest and so backed out. The remaining 814 (82.9%) subjects were used in the analysis.
Sample size selection
The researcher used Taro Yamane formula for sample selection (n = N ÷ 1 + N (0.05)2, n stands for sample size, N stands for the total population, 1 is constant, 0.05 stands for level of significance [20]. Therefore,
Therefore, the minimum sample size was 353
Inclusion criteria: Pregnant women who fulfilled the following study inclusion criteria were enrolled into the study: (1) attended and concluded their antenatal clinic at FMCO, (2) received standard malaria preventive treatment in pregnancy, (3) had no known underlying chronic illness, (4) those who gave their consent
Exclusion criteria: Women who were excluded included: (1) women who did not receive standard malaria preventive treatment in pregnancy, (2) those who did not conclude their ante natal visit or were not regular for ante natal at the hospital (3) those who came to receive other health care needs in the hospital, (4) mothers with multiple gestation, sickle cell disease, retroviral disease, and with any chronic ailment, (5) those who did not consent.
Validity of the instrument
Validity of the questionnaire, malaria determination, and birth weight were assured by the researcher’s supervisor, some other senior lecturers from Department of Nursing Science of Imo State University, Owerri, Nigeria.
Reliability of the instrument
The reliability of the instrument was assured by test-running the validated instrument, using 20 participants/pregnant women at Imo State Specialist Hospital, Owerri in a pilot study, whereby administration of questionnaires, fetal weight, and laboratory investigations were carried out as described by Amal, et al. [21]; Aguilar, Machevo and Mayor [22]; Uneke [23]; Bulmer, et al. [24].
Method of data collections
Data collection: Data collection involved administration of closed ended questionnaire to elicit information on biographic data. Clinical assessments/examinations (laboratory investigations) of maternal peripheral blood, and fetal birth weight were utilized.
Specimen Collection for laboratory investigation: To register all laboratory assessments, a data collection schedule form (DCSF) was used to record observations from clinical assessments (fetal birth weights) and laboratory investigations (maternal peripheral blood). The DCSF questionnaire was designed as described by Amal, et al. [21]; Salafia, Charles and Mass [25]; Bulmer, et al. [24]. Collection of sample too place from September 2020 to March 2021.
Questionnaire collection and administration process: The questionnaire only cover demographic characteristics of respondents e.g. age, gravidity, level of education, marital and occupational status. After receiving clearance from ethical committee of the hospital, the study was conducted using a paper questionnaire. A one-day training workshop was held for the six research assistants/data collectors (2 staff nurse midwife and 1 senior nursing officer each at the labour ward and antenatal clinic of the hospital) to orient them about the purpose of the study, the survey questionnaire and how to handle respondents. The researcher and/ senior nursing officer checked the copies of filled questionnaire at the end of each day for completeness. During ante natal visits/shortly before or immediately after child birth the questionnaire was used to obtain socio-demographic information from the participants. Detailed explanations of the content of the questionnaire was given to the participants by the researcher and/or the research assistants. Those participants who cannot read or understand were assisted.
Sample collection: Shortly before child birth maternal peripheral blood was obtained from each participant into sterile EDTA container for laboratory analysis [22,23]. The specimen were labeled and then sent to hematology laboratory where thick and thin films were prepared.
Sample processing/Examination of samples
Assessment of neonatal anthropometric parameters: Birth weights of babies were measured with a standard weighing scale [21] and categorized into two groups namely low birth weight babies defined as newborn babies weighing less than 2.5 kg (5.5 lbs) and normal newborn babies weighing 2.5 kg or more [25].
Peripheral blood microscopy: In hematology laboratory, thick smear of the blood specimen (maternal peripheral blood) were prepared on glass slides. The slides were allowed to dry and then stained with 3% Giemsa stain for 30minutes, rinsed with water and allowed to dry. The slides were then viewed under a microscope using oil immersion at x 100 magnification for presence of parasite [26]. Staining of slides and parasite counting were done by a medical laboratory scientist working in the hematology laboratory [27].
Determination of parasite density: Malaria diagnosis was based on identification of asexual stages of Plasmodium species on the thick blood film. Parasite density was determined by counting the number of parasites per high power field and ranged from + (1-10 parasites per 100 thick film fields), ++ (11-100 parasite per 100 thick film fields), +++ (1-10 parasites per single thick film field), and ++++ (more than 10 parasites per single thick film field). Two hundred high power fields examined before a slide were considered negative [23].
Data analysis
Statistical analysis of generated data was carried out using descriptive analysis and of percentages and presented using tables. Statistical comparisons and test of significance between positive and negative groups were calculated using the non-parametric Chi-square test. Differences were considered significant at P< 0.05.
Ethical clearance
The study protocol was reviewed and approved by the Ethical Clearance Committee of Federal Medical Centre, Owerri. Verbal consent was received from each participant before data collection. Respondents receive a detailed description of the research, confidentiality provisions and the fact that their participation will be voluntary and they could withdraw at any point if they so deemed. The principles of privacy and confidentiality was upheld. Two senior nursing officers from Department of Obstetrics unit and two senior laboratory technologist of Federal Medical Centre, Owerri were involved on the research. Also, two staff nurse/midwives who work at the labour ward of the hospital during the period of the study were involved onbirth weight assessment, and questionnaire administration and collection. All work were performed according to the guidelines for clinical research.
Results
Table 1 shows the socio-demographic characteristics of respondents. Out of 814 respondents, 61.9 % fall within the age brackets of 26–35 years, 19.3% and 18.2% make up16 – 25 years and 36 – 45 years age group respectively whereas 46 – 55 years constitute 0.6% of the population. For gravidity, Primigravida make up 30.8% of the population, 49.3% were multigravida; also grand multigravida and great-grand multigravida constitute 14.6% and 5.3% of the respondents respectively. For level of education, all the women went to school; 14.9% stopped at primary level of education, 48.7% had secondary education and 36.4% had tertiary education. For marital status, 5.3% were still single, 58.2% were still married, 22.1% and 14.4% were widowed and separated/divorced respectively. For occupational status, 12.5% were housewives/unemployed, 12.9% were students/apprentice, 36.6% were traders/farmers while 23.7% and 14.3% were self-employed and civil/public servants respectively Figure 1.
Figure 2 shows Prevalence of malaria according to maternal age groups, out of 157 respondents that fall within the age brackets of 16 – 25 years, 99 (63.1%) had malaria during pregnancy. Majority (70.2%) that are within the age brackets of 26 – 35 years had malaria whereas 65.6% and 60.0% of pregnant women of age brackets of 46 – 55 years and 36 – 45 years respectively had malaria. There is significant difference between malaria prevalence among pregnant women in FMC and their age groups (x2= 16.27; P < 0.05).
Figure 3 shows Prevalence of malaria according to gravidity, majority of Grand Multigravida, Great Grand Multigravida and Primigravida (73.1%, 72.1% and 71.3% respectively) had malaria whereas, 59.1% of Multigravida had malaria. There is significant difference between malaria prevalence among pregnant women in FMC and their gravidity status (x2 = 14.9; P < 0.05).
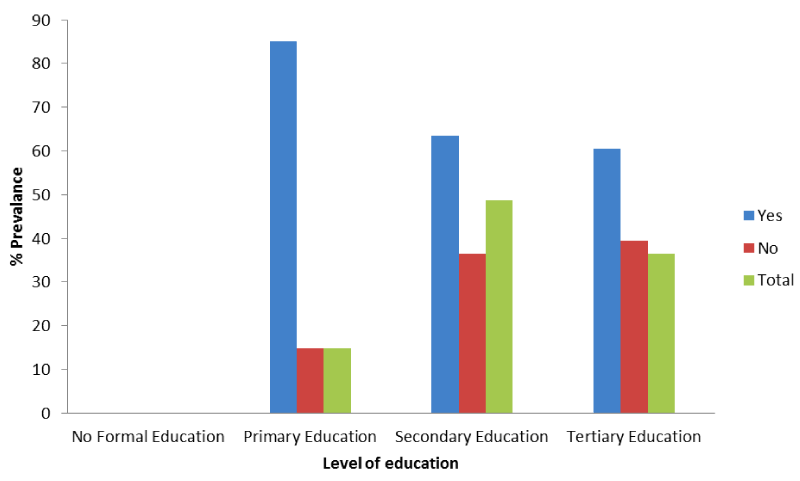
Figure 4 shows prevalence malaria according to level of education, a greater percent, 85.1% of 121 pregnant women that had only primary education had malaria whereas, 63.5% and 60.5% of those that stopped at secondary level (397) of education and those that had tertiary level (296) of education respectively had malaria. There is significant difference between malaria prevalence among pregnant women in FMC and their level of education (x2 = 24.69; P < 0.05).
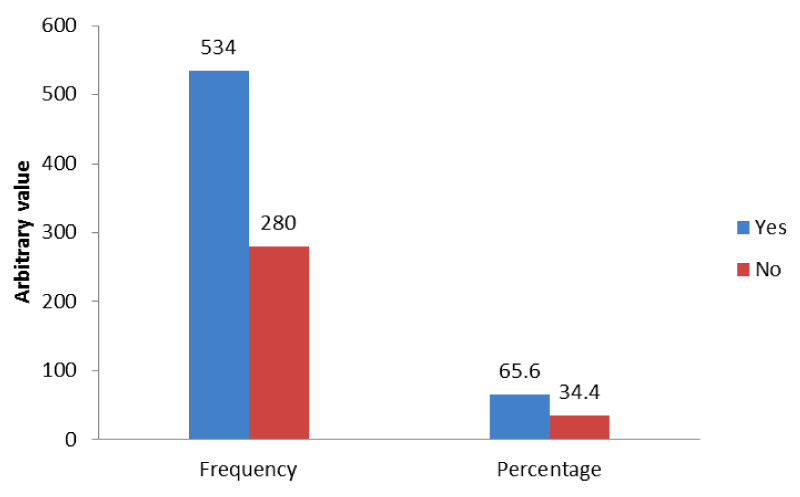
Table 2 shows overall prevalence of perinatal mortality and live births of pregnant women in FMC. Out of 534 pregnant women that had malaria within the period in FMC, 9.2% had stillbirth, 16.5% had miscarriage while 74.3% had life babies. Also out of 280 pregnant women with no malaria, 3.2% had stillbirth, 8.2% had miscarriage while 88.6% had life births. There is significant relationship between malaria and perinatal mortality among pregnant women in FMC, Owerri (x2 = 23.14; P < 0.05).
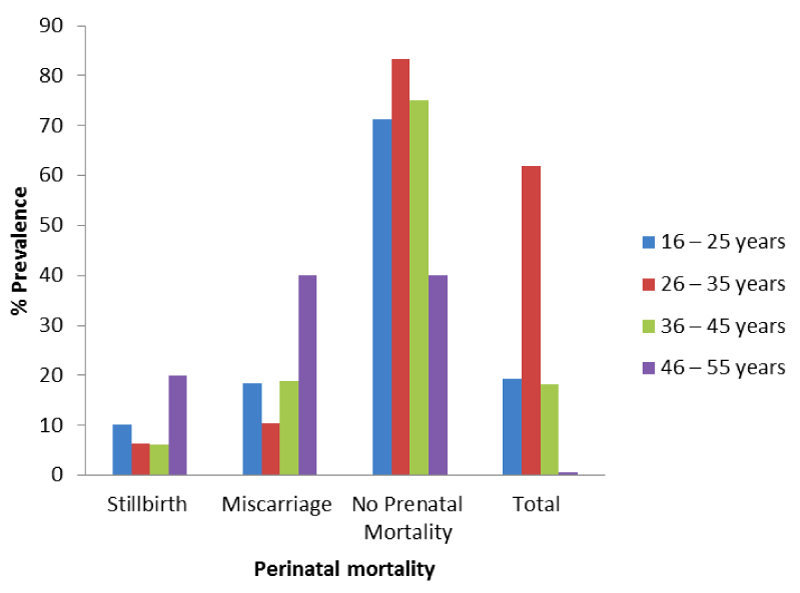
Figure 5 shows overall prevalence of perinatal mortality based on maternal age, out of 157 pregnant women that are within the age brackets of 16 – 25 years, 10.2% and 18.5% had stillbirths and miscarriage respectively. Also 504 respondents under age brackets of 26 – 35 years, 6.4% and 10.3% had stillbirth and miscarriage respectively. Those under the age groups of 36 – 45 years, 6.15 and 18.9% had stillbirth and miscarriage respectively whereas those that are under the age groups of 46 – 55 years, 20.0% and 40.0% had stillbirth and miscarriage respectively. There is significant relationship between perinatal mortality and maternal age groups among pregnant women in FMC, Owerri(x2 = 20.15; P < 0.05).
Table 3 shows the effect of malaria on prenatal mortality based on maternal age, out of 45 pregnant women within the age brackets of 16 – 25 years had perinatal mortality and 31.1% and 26.7% of them that had malaria had stillbirth and miscarriage respectively. Also pregnant women with age brackets of 26–35 years who had malaria, 31.0% and 52.4% had stillbirth and miscarriage respectively. A total of 37 women within age brackets of 36–45 years had perinatal mortality and 5.4% and 29.7% of them that had malaria had stillbirth and miscarriage respectively. Out of 3 women that are within age brackets of 46 -55 years had perinatal mortality but 66.7% of those that had malaria had miscarriage and 33.3% of those that do not have malaria had stillbirth. There is a significant effect of malaria on perinatal mortality based on maternal age among pregnant women in FMC, Owerri (x2= 40.11; P < 0.05). Table 4 shows overall prevalence of perinatal mortality based on gravidity, out of 251 primigravida women, 8.0% and 15.9% had stillbirths and miscarriage respectively. Also 401 multigravida women, 4.2% and 8.0% had stillbirth and miscarriage respectively. Those that are grand multigravida, 10.9 and 22.7% had stillbirth and miscarriage respectively whereas those that are great grand multigravida, 18.6% and 27.9% had stillbirth and miscarriage respectively. There is significant relationship between perinatal mortality and gravidity among pregnant women in FMC, Owerri (x2= 49.22; P < 0.05).
Table 5 shows the effect of malaria on perinatal mortality based on gravidity, out of 60 primigravida women that had perinatal mortality 30.0% and 28.3% of them that had malaria had stillbirth and miscarriage respectively. Also multigravida women who had malaria, 31.6% and 26.5% had stillbirth and miscarriage respectively. A total of 40 grand multiparous women that had perinatal mortality, 5.0% and 62.5% of them that had malaria had stillbirth and miscarriage respectively. Out of 20 great grand multiparous women that had perinatal mortality,25.0% and 50.0% of them that had malaria had stillbirth and miscarriage respectively. There is a significant effect of malaria on perinatal mortality based on gravidity among pregnant women in FMC, Owerri (x2= 48.67; P < 0.05).
Figure 6 shows the overall prevalence of preterm delivery among pregnant women in FMC, Owerri. Out of 645 of pregnant women that had life birth, 397 (61.6%) of them had malaria with 26.2% of the women having preterm delivery whereas of 248 that do not have malaria, 9.3% had preterm delivery. A total of 127 (19.7%) of pregnant women in FMC, Owerri had preterm delivery. There is significant relationship between malaria and preterm delivery among pregnant women in FMC, Owerri (x2 = 27.58; P < 0.05).
Table 6 shows effect of malaria on preterm delivery based on maternal age; 21.4% of pregnant women within age brackets of 16 – 25 years that had malaria had preterm delivery whereas 7.1% of those that do not have malaria had preterm delivery. Also 16.4% of pregnant women of 26 – 35 age groups who had malaria had preterm delivery and 2.4 % of the same age group who had no malaria had preterm delivery. Age groups of 36 – 45 years, 9.0% of those that had malaria had preterm delivery whereas 3.6% of those that do not have malaria under same age group had preterm delivery. Moreover, 50.0% of pregnant women under age brackets of 46 – 55 years who had malaria had preterm delivery while same 50.0% of them who had no malaria had preterm delivery. Statistically, malaria significantly affects preterm delivery based on maternal age (x2 = 49.2; P < 0.05).
Table 7 shows effect of malaria on preterm delivery based on gravidity; 22.5% of primigravida women who had malaria had preterm delivery whereas 4.2% of those that do not have malaria had preterm delivery. Also 12.8 % of multiparous women who had malaria had preterm delivery and 2.8 % of the same gravidity who had no malaria had preterm delivery. For grand multigravida, 10.1% of those that had malaria had preterm delivery whereas 6.4% of those that do not have malaria under same gravidity had preterm delivery. Moreover, 34.8% of great grand multiparous women who had malaria had preterm delivery while none of same gravidity who had no malaria had no preterm delivery. Statistically, malaria significantly affects preterm delivery based on gravidity (x2 = 56.94; P < 0.05).
Figure 7 shows the effect of malaria on birth weight among pregnant women in FMC, Owerri. Out of 645 of pregnant women that had life birth, 397 (61.6%) of them had malaria with 15.9% of the women having low birth weight babies whereas of 248 (38.4%) that do not have malaria, 35.9% of them had low birth weight babies. A total of 152 (23.6%) of pregnant women in FMC, Owerri had low birth weight babies. Statistically, malaria significantly affects birth weight among pregnant women in FMC, Owerri (x2 = 34.06; P < 0.05).
Table 8 shows effect of malaria on birth weight based on maternal age; 2.7% of pregnant women within age brackets of 16 – 25 years that had malaria had low birth weight whereas 8.9% of those that do not have malaria had low birth weight. Also 12.1 % of pregnant women of 26 – 35 age groups who had malaria had low birth weight and 13.8 % of the same age group who had no malaria had low birth weight. Age groups of 36 – 45 years, 6.3% of those that had malaria had low birth weight whereas 18.9% of those that do not have malaria under same age group had low birth weight. Moreover, all the pregnant women under age brackets of 46 – 55 years who had malaria had low birth weight. Statistically, malaria significantly affects low birth weight based on maternal age (x2 = 53.82; P < 0.05).
Table 9 shows effect of malaria on birth weight based on gravidity; 5.8% of primigravida women who had malaria had low birth weight whereas 21.5% of those that do not have malaria had low birth weight. Also 12.5 % of multiparous women who had malaria had low birth weight and 10.5 % of the same gravidity who had no malaria had low birth weight. For grand multigravida, 3.8% of those that had malaria had low birth weight whereas 5.0% of those that do not have malaria under same gravidity had low birth weight. Moreover, 21.8% of great grand multiparous women who had malaria had low birth weight while 30.4% of same gravidity who had no malaria had low birth weight. Statistically, malaria significantly affects preterm delivery based on gravidity (x2 = 65.94; P < 0.05).
Discussion
Majority (61.9%) of pregnant women in FMC, Owerri were under the age brackets of 26 – 35 years, 49.3% were multiparous, 48.7% and 36.4% had only secondary and tertiary education respectively. More than half (58.2%) were married.
Prevalence of malaria among pregnant women in Federal Medical Centre, Owerri, Imo State, Nigeria
The study revealed that 534 (65.6%) of pregnant women in FMC, Owerri had peripheral malaria. Majority (70.2% and 73.1%) of age brackets of 26 – 35 years and grand multiparous women had malaria respectively. Also, 85.1% of the women that had malaria had only primary education. The study further revealed that malaria is significantly associated with age (x2 = 16.27; P < 0.05), gravidity (x2 = 14.9; P < 0.05), and level of education (x2 = 24.69; P < 0.05). This finding correlates with report of Adefioye, et al. [28] on prevalence of malaria parasite infection among pregnant women in Osogbo, Southwest Nigeria which showed a very high (72%) prevalence of malaria parasite in their blood. This finding contradicts with report of Uko, Emeribe and Ejezie [29] who recorded low prevalence of (31.8%). It is also in contrast with studies carried out in sub-Saharan Africa between 2000 and 2011 by Desai, Gutman and L’lanziva (2015) were prevalence of malaria in pregnant women attending antenatal clinics was 29.5% in East and Southern Africa and 35.1% in West and Central Africa. The discrepancy in the result was because of the time/period (season of year i.e. rainy season when mosquito multiplication is higher) when this study and that of Adefioye, et al. [28] carried out their studies as against the study of Uko, Emeribe and Ejezie (2014). Regardless of symptoms, the presence of plasmodial parasites in a pregnant woman’s body will have a negative impact on her own health and that of her fetus. Restricting treatment to symptomatic pregnant women is an inadequate strategy to reduce the morbidity and mortality associated with malaria (Nosten, McGready and Mutabingwa, 2007).
According to Takem and D’Alessandro (2013) subclinical infection is common in areas where natural immunity is high (e.g., sub-Saharan Africa), whereas symptomatic cases are more common in areas with low immunity (e.g., the Asia-Pacific region, and South Africa). Malaria in pregnancy is different to the disease in the non-pregnant state. The severity of malaria in pregnancy is thought to be due to general impaired immunity plus a diminution of acquired immunity to malaria in endemic areas.
In areas where malaria is highly endemic, a protective semi-immunity against Plasmodium falciparum is acquired during the first 10 to 15 years of life, and the majority of malaria-related morbidity and mortality occur in young children. However, in contrast with low malaria prevalence in adults, pregnant women in endemic areas are highly susceptible to malaria, and both the frequency and the severity of disease are higher in pregnant women (Meeusen, et al. 2010). In pregnancy, there is a transient depression of cell-mediated immunity that allows fetal allograft retention but also interferes with resistance to various infectious diseases. Furthermore, cellular immune responses to Plasmodium falciparum antigens are depressed in pregnant women [30].
Effect of Malaria on perinatal mortality among pregnant women in Federal Medical Centre, Owerri, Imo State, Nigeria
A total of 169 (20.8%) of the women had perinatal mortality, 58 (7.1%) had stillbirth and 111(13.7%) had miscarriage. This shows that a total of 645 (79.2%) had life babies. There is also significant relationship between malaria and perinatal mortality among pregnant women in FMC, Owerri (x2= 23.14; P < 0.05). The finding of this study correlates with the finding of [17] whose finding revealed malaria as a cause of perinatal death (including stillbirth) in Africa.
The study further revealed that perinatal mortalities were high among great grand multigravida (18.6%) and women with age bracket of 46 – 55 years (20.0%). Further analysis also revealed that there is significant effect of malaria on perinatal mortality based on gravidity (x2 = 48.67; P < 0.05) and maternal age (x2 = 40.11; P < 0.05). This finding is in line with a study in Zaire, Nigeriawhich found out that maternal peripheral infection significantly increased the risk of perinatal death [31]. Fetal mortality is estimated at 15% for P. vivax and around 30% for P. falciparum. Malaria is significantly associated with gravidity and age of the women [32]. According to Seal, Mukhopadhay and Ganguly (2010) common problems for the fetus whose mother are malaria positive include, spontaneous abortion, stillbirth, premature delivery, intrauterine growth restriction, low birth weight, intrauterine fetal death etc. Maternal infection can also be associated with missed abortion, preterm labour, intrauterine growth restriction and intrauterine fetal death [33].
Effect of malaria on preterm delivery among pregnant women in Federal Medical Centre, Owerri, Imo State, Nigeria
The study revealed an overall prevalence or preterm delivery of 19.7%. There is also a significant relationship between malaria and preterm delivery among pregnant women in Federal Medical Centre, Owerri, Imo State, Nigeria (x2 = 27.58; P < 0.05). Malaria presents a significant impact on the neonates, being associated with increased risk of spontaneous abortion, stillbirth, premature delivery, fetal death, Low Birth Weight (LBW) and fetal/child development retardation in malaria-endemic countries [10,34]. Malaria is also significantly associated with preterm delivery and intra uterine growth retardation [14].
The finding further revealed that maternal age of 46- 55 and great grand multiparous women experience preterm deliveries of 50.0% and 34.8% more than others respectively. Also there is significant effect of malaria on preterm delivery based on maternal age (x2 = 49.2; P < 0.05) and gravidity (x2 = 56.94; P < 0.05). The finding of this study correlates with that of De Beaudrap, Turyakira and White [31] which revealed a significant association between the risk of pre-term delivery and the occurrence of a malaria infection among the study group. Malaria in pregnancy not only affects the mother but also has a dangerous sequel for the developing fetus, resulting in premature delivery or intrauterine growth retardation [19].
Effect of malaria on fetal birth weight among pregnant women in Federal Medical Centre, Owerri, Imo State, Nigeria
The study revealed that 23.6% of pregnant women in FMC, Owerri had low birth weight babies. Furthermore, malaria significantly affects birth weight among pregnant women in FMC, Owerri (x2 = 34.06; P < 0.05). This finding correlates with a study of Bardaji, Sigaugue and Menendez [35] who found out that low birth weight, prematurity and risk of dying during infancy was increased among infants born to women with acute peripheral and placental malaria infection. Malaria during pregnancy can result in low birth weight (LBW), an important risk factor for infant mortality [13].
The study further revealed that there is a significant effect of malaria on birth weight based on maternal age (x2 = 53.82; P < 0.05) and gravidity (x2 = 65.94; P < 0.05). Malaria infection during pregnancy can have adverse effects on both mother and fetus, including maternal anemia, fetal loss, premature delivery, intrauterine growth retardation, and delivery of low birth-weight infants (<2500 g or <5.5 pounds), a risk factor for death. The risk is also associated with maternal age and gravidity [33]. Malaria infection during pregnancy has been said to cause infant mortality indirectly through its contribution to low birth weight and premature delivery, and it has been estimated that it would be responsible for 75,000–200,000 infant deaths in the sub-Saharan region [16,36,37]. On the other hand, a study in Zaire found that maternal peripheral infection significantly increased the risk of low birth weight and perinatal death [31].
In contrast to this study, some studies have examined this association. Two studies conducted in Sudan and Uganda, respectively, found that peripheral malaria infection during pregnancy was not associated with low birth weight and increased infant mortality [38,39]. Similarly, another study in Kenya found that peripheral and placental malaria was not associated with post neonatal mortality in both HIV-positive and HIV-negative mothers [40]. The contrast might be from area of study, sample size, time of study and method of data analysis [41-98].
Summary of findings
The present work was able to establish:
A: Peripheral Malaria Prevalence
1. The overall prevalence of malaria parasite infection in the study population was 65.6%.
2. Malaria prevalence was significantly higher (70.2%) among maternal age group of 26-35 years than others.
3. Malaria prevalence was significantly higher (73.1%) in grand multigravidae than other gravidities.
4. Those with the least level of education (primary) had more malaria prevalence (85.1%) than other levels of education.
5. Malaria prevalence is significantly associated with maternal age bracket (x2 = 16.27; P < 0.05), gravidity (x2= 14.9; P < 0.05) and level of education (x2 = 24.69; P < 0.05).
B: Effect of Malaria on Perinatal Mortality
1. Perinatal Mortalities were group into two (Stillbirths and Miscarriages) which recorded 7.1% and 13.7% respectively.
2. There is significant relationship between malaria and perinatal mortality (x2 = 23.14; P < 0.05).
3. Perinatal mortalities of pregnant women with malaria were higher (31.1%) in maternal age groups of 16 – 25 years than other age groups
4. Also multigravidae women who had malaria have higher perinatal mortality (30.6%) than other gravidities
5. There is significant effect of malaria on perinatal mortality based on maternal age (x2 = 40.11; P < 0.05)and gravidity (x2= 48.67; P < 0.05)
C. Effect of Malaria on Preterm Delivery
1. An overall prevalence of preterm deliveries were 19.7%.
2. There is significant relationship between malaria and preterm delivery (x2 = 27.58; P < 0.05).
3. Preterm deliveries for those with malaria were higher (50.0%) in maternal age groups of 46 – 55 years than other age groups
4. Also great grand multigravidae women who had malaria have higher preterm deliveries (34.8%) than other gravidities
5. There is significant effect of malaria on preterm delivery based on maternal age (x2 = 49.2; P < 0.05)and gravidity (x2 = 56.94; P < 0.05).
D. Effect of Malaria on Fetal Birth Weight
1. An overall prevalence of low birth weight were 23.6%.
2. There is significant relationship between malaria and fetal birth weight (x2 = 34.06; P < 0.05).
3. All pregnant women within age brackets of 46 – 55 years who had malaria had low birth weight babies.
4. Also great grand multigravidae women who had malaria have higher low birth weight babies (21.8%) than other gravidities
5. There is significant effect of malaria on fetal birth weight based on maternal age (x2 = 53.82; P < 0.05) and gravidity (x2 = 65.94; P < 0.05)
Conclusion
This study assessed the prevalence of malaria and its adverse fetal outcomes in Federal Medical Centre (FMC), Owerri, Imo State, Nigeria. The prevalence of malaria among pregnant women was found to be relatively high. Also malaria prevalence among the pregnant women were significantly associated with gravidity, gender and level of education. The study further revealed a significant effect of malaria on perinatal mortality, preterm delivery and birth weight based on gender and gravidity.
Recommendations
1. Malaria is largely a major public health concern with its resultant effect seen in this study population. The study suggests that any meaningful control measures in pregnancy should start as early as possible to curb the menace of miscarriages and still birth.
2. This study indicated that peripheral malaria is still high among the study population and calls for the intensified efforts in malaria control in pregnancy.
3. The study suggests effective therapy since perinatal mortality due to malaria was recorded in this study
4. Preterm deliveries and low fetal birth weight based on gravidity and maternal age groups associated with malaria as identified is a call for program managers to make haste and implement new strategies for malaria control. For example, use of rapid diagnostic tests to screen women for malaria at the first or each antenatal visit and treatment of positive women with artemisinin combination therapies.
5. As part of public health measures, there is the need for effective linkages between malaria control and antenatal care programmes in order to improve the success of efforts to control malaria during pregnancy.
6. To reduce the burden of malaria infection in pregnancy, there is need to adhere to a three-pronged approach recommended by the World Health Organization (World Health Organization, 1992; World Health Organization; 1997) these include; use of intermittent preventive treatment (IPT), insecticide-treated nets (ITN), and case management of malaria illness.
- Yartey JE (2016) Malaria in pregnancy: Access to effective interventions in Africa. International Journal of Gynecology and Obstetrics 94: 364–373. Link: https://bit.ly/2VBDPGS
- Taylor DW, Zhou A, Marsillio LE, Thuita LW, Leke EB, et al. (2014) “Antibodies That Inhibit Binding of Plasmodium falciparum-Infected Erythrocytes to Chondroitin Sulfate A and to the C Terminus of Merozoite Surface Protein 1 Correlate with Reduced Placental Malaria in Cameroonian Women. Infect Immun 72: 1603–1607. Link: https://bit.ly/2U2EmRS
- World Health Organization (2017) World malaria report 2016. Link: https://bit.ly/3AjYM8u
- World Health Organization (2018) “Implementing malaria in pregnancy programs in the context of World Health Organization recommendations on antenatal care for a positive pregnancy experience,” Link: https://bit.ly/3xp61di
- World Health Organization (2007) Gender, health and malaria. Health Policy (New York) 52: 267–292. Link:
- Marchesini P, Crawley J (2014) Reducing the burden of malaria in pregnancy. MERA IV, supporting agency–Roll Back Malaria.
- Malaria consortium (2016) Malaria control Nigeria: State fact sheets. Link: https://bit.ly/3fG8B8K
- Tako EA, Zhou A, Lohoue J, Leke R, Taylor DW, et al. (2008) Risk factors for placental malaria and its effect on pregnancy outcome in Yaoundé, Cameroon. Journal of Tropical Medical Hygiene 72: 236–242.
- Heggenhougen HK, Hackethal V, Vivek P (2003) The behavioural and social aspects of malaria control: An introduction and annotated bibliography. WHO on behalf of the Special Programme for Research and Training in Tropical Diseases 3. Link: https://bit.ly/37ofCGP
- Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, et al. (2007) Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 7: 93-104. Link: https://bit.ly/3AnesYj
- Morgan HG (2014) Placental malaria and low birthweight neonates in urban Sierra Leone. Trop Med Parasitol 88: 575–580.
- Guyatt HL, Snow RW (2001) The epidemiology and burden of Plasmodium falciparum-related anemia among pregnant in sub-Saharan Africa. Journal of Tropical Medical Hygiene 64: 36–44. Link: https://bit.ly/3CqDs2P
- Guyatt HL, Snow RW (2014) Impact of malaria in Africa. Clinical Microbiology Review 17: 760-769. Link:
- Ohalete CN, Dozie INS, Nwachukwu MI, Obiukwu CE (2011) Epidemiology and Economic consequences of malaria in pregnant womenin Imo State, Nigeria. African Journal of Microbiology Research 5: 3895-3900. Link: https://bit.ly/3iq2x66
- Steketee RW (2006) Malaria prevention in pregnancy: the effects of treatment and chemoprophylaxis on placental malaria infection, low birth weight, and fetal, infant, and child survival. Mangochi Malaria Research Project. Washington, D.C.: United States Agency for International Development.
- Menendez C, Ordi J, Ismail MR (2000) The impact of placental malaria on gestational age and birth weight. J Infect Dis 181: 1740–1745. Link: https://bit.ly/3yvEtV0
- Shulman CE, Dorman EK (2007) Reducing childhood mortality in poor countries ― Importance and prevention of malaria in pregnancy. Journal of Tropical Medical Hygiene 7: 30–35.
- Akanbi OL (2013) Effect of malaria infection on oxidative stress and lipid profile in pregnant women. Journal of Medical Sciences 4: 128-133. Link: https://bit.ly/37sPz0Z
- Fleming AF (2009) The aetiology of severe malaria in pregnancy in Ndola, Zambia. Ann Trop Med Parasitol 83: 37–49. Link: https://bit.ly/2VwQLxM
- Onyeneke EN, Amadi JC, Anyanwu HO, Ekeogu EC, Enyoh CE (2020) Food, Health Habits and Anthropometric Indices of Under-Five Aged Children in Imo State, Nigeria. Journal of Food Nutrition and Metabolism 3: 1-9. Link: https://bit.ly/3lH2uoj
- Amal HM, Magdi MS, Elhassan ME, Ahmed AM, Salah EE, et al. (2013) Submicroscopic Plasmodium Falciparum Malaria and low birth weight in Central Sudan. Malaria Journal 12: 172–178. Link: https://bit.ly/3itI2W7
- Aguilar R, Machevo S, Menéndez C, Bardají A, Nhabomba A, et al. (2012) Comparison of placental blood microscopy and the ICT HRP2 rapid diagnostic test to detect placental malaria. Trans R Soc Trop Med Hyg 106: 573-575. Link: https://bit.ly/3fI3Ifl
- Uneke CJ (2008) Diagnosis of Plasmodium falciparum variant surface antigens in protective immunity and vaccine development. Human Vaccine 6: 84- 89.
- Bulmer JN, Rasheed FN, Francis N, Morrison L, Greenwood BM (2003) Placental malaria I: Pathological classification. Histopathology 22: 211–218. Link: https://bit.ly/3rYfUxv
- Salafia CM, Charles AK, Mass EM (2006) Placenta and fetal growth restriction. Clin obstet Gynecol 49: 236–256. Link: https://bit.ly/3xuRrAU
- Mayor A, Moro L, Aguilar R, Bardají A, Cisteró P, et al. (2012) How hidden can malaria be in pregnant women? Diagnosis by microscopy, placental histology, polymerease chain reaction and detection of histidine-rich protein 2 in plasma. Clin Infect Dis 54: 1561–1568. Link: https://bit.ly/3iumiJS
- Murray CK, Grasser RA, Magill AJ, Miller RS (2008) Update on rapid diagnostic testing for malaria. Clin Microbiol Rev 21: 97–100. Link: https://bit.ly/3rZ3fKE
- Adefioye OA, Adeyeba OA, Hassan WO, Oyeniran OA (2007) Prevalence of Malaria Parasite Infection among Pregnant Women in Osogbo, Southwest, Nigeria. American-Eurasian Journal of Scientific Research 2: 43-45. Link: https://bit.ly/3s3H463
- Uko EK, Emeribe AO, Ejezie GC (1998) Malaria Infection of the Placenta and Neonatal Low Birth Weight in Calabar. Journal of Medical Laboratory Sciences 7: 7-10.
- Riley EM, Schneider G, Sambou I, Greenwood BM (2008) Suppression of cell-mediated immune responses to malaria antigens in pregnant Gambian women. Am J Trop Med Hyg 40: 141–144. Link: https://bit.ly/3AnfK5B
- Nyirjesy P, Kavasya T, Axelrod P, Fischer PR (2013) Malaria during pregnancy: neonatal morbidity and mortality and the efficacy of chloroquine chemoprophylaxis. Clin Infect Dis 16: 127–132. Link: https://bit.ly/2U2FwwI
- Brabin BJ, Agbaje SOF, Ahmed Y, Briggs ND (2009) A birthweight nomogram for Africa, as a malaria-control indicator. Ann Trop Med Parasitol 111: 77-83. Link: https://bit.ly/3An5M4a
- Centers for Disease Control and Prevention (2015) Intermittent Preventive Treatment of Malaria for Pregnant Women (IPTp)
- Rogerson SJ, Van den Broek NR, Chaluluka E, Qongwane C, Mhango CG, et al. (2000) Malaria and anemia in antenatal women in Blantyre, Malawi: a twelve-month survey. Am J Trop Med Hyg 62: 335–340. Link: https://bit.ly/3jyyUio
- Bardaji A, Sigaugue B, Sanz S, Maixenchs M, Ordi J, et al. (2011) Impact of malaria at the end of pregnancy on infant mortality and morbidity. J Infect Dis 203: 691-699. Link: https://bit.ly/3iuLqA5
- Greenwood BM, Greenwood AM, Snow RW, Byass P, Bennett S, et al. (2018) The effects of malaria chemoprophylaxis given by traditional birth attendants on the course and outcome of pregnancy. Tropical Medicine and Hygiene 83: 589–594.
- Romagosa C, Ordi J, Saute F, Quintó L, Machungo F, et al. (2017) Seasonal variations in maternal mortality in Maputo, Mozambique: the role of malaria. Trop Med Int Health 12: 62-67. Link: https://bit.ly/2TYLv5x
- Brahmbhatt H, Sullivan D, Kigozi G, Askin F, Wabwire-Mangenm F, et al. (2018) Association of HIV and malaria with mother-to-child transmission, birth outcomes, and child mortality. J Acquir Immune Defic Syndr 47: 472–476. Link: https://bit.ly/3itW36j
- Haghdoost AA, Alexander N, Smith T (2007) Malaria during pregnancy and infant mortality rate: critical literature review and a new analytical approach. J Vector Borne Dis 44: 98–104. Link: https://bit.ly/3rXQm3F
- van Eijk AM, Ayisi JG, Ter Kuile FO, Slutsker L, Shi YP, et al. (2007) HIV, malaria, and infant anemia as risk factors for postneonatal infant mortality among HIV-seropositive women in Kisumu, Kenya. Journal of Infectious Diseases 196: 30–37. Link: https://bit.ly/3lGEfHa
- Achidi EA, Kuoh AJ, Minang JT, Ngum B, Achimbom BM, et al. (2005) Malaria infection in pregnancy and its effects on haemoglobin levels in women from a malaria endemic area of Fako Division, South West Province, Cameroon. J Obstet Gynaecol 25: 235–240. Link: https://bit.ly/3AoXbyd
- Anagnos D, Lanoie LO, Palmieri JR, Ziefer A, Connor DH (2005) Effects of placental malaria on mothers and neonates from Zaire. Z Parasitenkd 72: 57–64. Link: https://bit.ly/3AiDhor
- Antelman G, Msamanga GI, Spiegelman D, Urassa EJ, Narh R, et al. (2000) Nutritional factors and infectious disease contribute to anemia among pregnant women with human immunodeficiency virus in Tanzania. J Nutr 130: 1950–1957. Link: https://bit.ly/3An6Jtg
- Asmelash D, Ambachew S, Eshetie S, Addisu A, Zeleke AJ (2019) The prevalence of malaria among pregnant women in Ethiopia: A systematic Review and Meta-analysis. Journal of Parasitology 2019: 8396091. Link: https://bit.ly/3lH48Gv
- Bloland PB, Wirima JJ, Steketee RW, Chilima B, Hightower A, et al. (2005) Maternal HIV infection and infant mortality in Malawi: evidence for increased mortality due to placental malaria infection. AIDS 9: 721–726. Link: https://bit.ly/3jwM92X
- Bouyou-Akotet MK, Ionete-Collard DE, Mabika-Manfoumbi M, Kendjo E, Matsiegui PB, et al. (2003) Prevalence of Plasmodium falciparum infection in pregnant women in Gabon. Malar J 2: 18. Link: https://bit.ly/3fHpHTH
- Brabin BJ (2004) The risks and severity of malaria in pregnant women. Geneva: WHO; 2003.
- Brabin BJ (1983) An analysis of malaria in pregnancy in Africa. Bull World Health Organ 61: 1005–1016. Link: https://bit.ly/3CqFQqb
- Brabin BJ, Verhoeff F (2006) The contribution of malaria. In: Mac-Lean AB, Neilson JP, editors. Maternal Morbidity and Mortality. London: RCOG Press 65–78. Link:
- Bugvi SM, Ahmed N (2015) Congenital malaria: A rare entity. J Coll Physicians Surg Pak 25: 841-842. Link: https://bit.ly/2VA8SD9
- De Beaudrap P, Turyakira E, White LJ, Nabasumba C, Tumwebaze B, et al. (2013) Impact of malaria during pregnancy on pregnancy outcomes in a Ugandan prospectivecohort with intensive malaria screening and prompt treatment. Malar J 12: 139. Link: https://bit.ly/37r1Kf2
- Dicko A, Mantel C, Aly-Thera M, Doumbia S, Diallo M, et al. (2003) Risk factors for malaria infection and anemia for pregnant women in the Sahel area of Bandiagara, Mali. ActaTrop 89: 17-23. Link: https://bit.ly/3AeHAky
- Fondjo LA, Addai Mensah O, Annani-Akollor ME, Quarshie JT, Boateng AA, et al. (2020) A multicenter study of the prevalence and risk factors of malaria and anemia among pregnant women at first antenatal care visit in Ghana. Plos One 15: e0238077. Link: https://bit.ly/3yxr5Q6
- Fried M, Duffy PE (2017) Malaria during Pregnancy. Cold Spring Harb Perspect Med 7: a025551. Link: https://bit.ly/3yvp47h
- Getachew M, Yewhalaw D, Tafess K, Getachew Y, Zeynudin A, et al. (2012) Anaemia and associated risk factors among pregnant women in Gilgel Gibe dam area, Southwest Ethiopia. Parasit Vectors 5: 296. Link: https://bit.ly/3jCV4QG
- Githeko AK, Mbogo CNM, Curtis CF, Lines J, Lengeler C (2011) Entomological monitoring of large-scale vector control interventions. Parasitology Today 44: 101-107. Link: https://bit.ly/3lG7V6Y
- Hack M, Klein N, Taylor HG (2013) Long-term developmental outcomes of low-birth weight infants. Future Child 25: 333-339.
- Hahn S, Williamson PR, Hutton JL, Garner P, Garner P (2000) Assessing the potential for bias in meta-analysis due to selective reporting of subgroup analyses within studies. Stat Med 19: 3325-3336. Link: https://bit.ly/3jA9noQ
- Hanscheid T (2009) Diagnosis of malaria: a review of alternatives to conventional microscopy. Clin Lab Haematol 28: 186–209.
- Hoestermann CF, Ogbaselassie G, Wacker J, Bastert G (2004) Maternal mortality in the main referral hospital in The Gambia, West Africa. Trop Med Int Health 1: 710-717. Link: https://bit.ly/3xsSi5m
- Institute of Medicine (1985) Preventing low birthweight. National Academy Press, Washington, D.C. Link: https://bit.ly/3lF6DsZ
- Ismail MR, Ordi J, Menendez C, Ventura PJ, Aponte JJ, et al. (2010) Placental pathology in malaria: a histological, immunohistochemical, and quantitative study. Hum Pathol 31: 85–93. Link: https://bit.ly/3jpBIy0
- Johnson AH, Leke RG, Mendell NR, Shon D, Suh YJ, et al. (2009) HLA class II alleles influence levels of antibodies to the Plasmodium falciparum asexual-stage apical membrane antigen 1 but not to merozoite surface antigen 2 and merozoite surface protein 1. Infect Immun 72: 2762–2771. Link: https://bit.ly/3lFBd5R
- Le Hesran JY, Cot M, Personne P, Fievet N, Dubois B, et al. (1997) Maternal placental infection with Plasmodium falciparum and malaria morbidity during the first 2 years of life. Am J Epidemiol 146: 826-831. Link: https://bit.ly/3iqY4jE
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, et al. (2004) Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350: 672-683. Link: https://bit.ly/2VvvwfL
- Malhotra I, Dent A, Mungai P, Wamachi A, Ouma JH, et al. (2009) Can prenatal malaria exposure produce an immune tolerant phenotype? A prospective birth cohort study in Kenya. PLoS Med 6: e1000116. Link: https://bit.ly/3jtVA3f
- Mangochi Malaria Research Project (1993) Malaria prevention in pregnancy: the effects of treatment and chemoprophylaxis on placental malaria infection, low birth weight, and fetal, infant, and child survival. U.S. Agency for International Development in conjunction with Centers for Disease Control and Prevention, Atlanta, Ga.
- Matteelli A, Donato F, Shein A, Muchi JA, Leopardi O, et al. (2010) Malaria and anemia in pregnant women in urban Zanzibar, Tanzania. Tropical Medical Parasitology 88: 475-483.
- Maubert B, Fievet N, Tami G, Cot M, Boudin C, et al. (2009) Development of antibodies against chondroitin sulfate A-adherent Plasmodium falciparum in pregnant women. Infect Immun 107: 869-875.
- Maynard SE, Min JY, Merchan J, Lim KH, Li J, et al. (2003) Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658. Link: https://bit.ly/3CqK2Gz
- McCormick MC (2015) The contribution of low birth weight to infant mortality and childhood mortality. N Engl J Med 443: 91-99.
- McCormick MC, Brooks-Gunn J, Workman-Daniels K, Turner J, Peckham GJ (1992) The health and developmental status of very low-birth-weight children at school age. JAMA 267: 2204-2208. Link: https://bit.ly/3xqag8l
- McGregor IA (2005) Epidemiology, malaria, and pregnancy. Journal of Tropical Medical Hygiene 33: 517–525.
- McGregor IA, Wilson ME, Billewicz WZ (2004) Malaria infection of the placenta in the Gambia, West Africa: its incidence and relationship of stillbirth, birthweight, and placental weight. Journal of Tropical Medical Hygiene 77: 232–244.
- Muehlenbachs A, Mutabingwa TK, Edmonds S, Fried M, Duffy PE (2006) Hypertension and maternal–fetal conflict during placental malaria. Journal of Tropical Medical Hygiene 79: 398-446.
- Mutabingwa TK (2015) Artemisinin-based combination therapies (ACTs): best hope for malaria treatment but inaccessible to the needy! Acta Trop 95: 305-315. Link: https://bit.ly/3yvRi1D
- Newman RD, Steketee RW, Parise ME (2013) Burden of malaria during pregnancy in areas of stable and unstable transmission in Ethiopia during a nonepidemic year. J Infect Dis 210: 1882-1886.
- Nokes C, van den Bosch C, Bundy DAP (2008) The effects of iron deficiency and anemia on mental and motor performance, educational achievement and behaviour in children: an annotated bibliography. Washington, D.C.: International Nutritional Anemia Consultative Group.
- Oeuvray C, Theisen M, Rogier C, Trape JF, Jepsen S, et al. (2006) Cytophilic immunoglobulin responses to Plasmodium falciparum glutamate-rich protein are correlated with protection against clinical malaria in Dielmo, Senegal. Infect Immun 68: 2617-2620.
- Okoko BJ, Ota MO, Yamuah LK, Idiong D, Mkpanam SN, et al. (2002) Influence of placental malaria infection on foetal outcome in the Gambia: twenty years after Ian McGregor. J Hlth Pop Nutr 20: 4-11. Link: https://bit.ly/37ocVFh
- Parise ME, Sambo C (2006) The burden of malaria in pregnancy in malaria-endemic areas. Journal of Tropical Medical Hygiene 64: 28-35.
- Pertet AM (1990) The effect of weekly chloroquine prophylaxis given to pregnant mothers and their respective infants on birthweight and physical growth during infancy in a rural area of Kenya. Ph.D. thesis. University of Nairobi, Nairobi, Kenya.
- Rogerson SJ, Desai M, Mayor A, Sicuri E, Taylor SM, et al. (2018) Burden, pathology, and costs of malaria in pregnancy: new developments for an old problem. Lancet Infect Dis 18: 5-9. Link: https://bit.ly/3xxuiho
- Salihu HM, Tchuinguem G, Ratard R (2012) Effect of chloroquine prophylaxis on birthweight and malaria parasite load among pregnant women delivering in a regional hospital in Cameroon. West Indian Med J 99: 238-244.
- Scherf A, Pouvelle B, Buffet PA, Gysin J (2007) Molecular mechanisms of Plasmodium falciparum placental adhesion. Journal of Tropical Medical Hygiene 3: 125.
- Schwarz NG, Adegnika AA, Breitling LP, Gabor J, Agnandji ST, et al. (2008) Placental malaria increases malaria risk in the first 30 months of life. Clin Infect Dis 47: 1017-1025. Link: https://bit.ly/3lIVlE2
- Seal SL, Mukhopadhay S, Ganguly RP (2010) Malaria in pregnancy. J Indian Med Assoc 108: 487-490. Link: https://bit.ly/3fJBvF3
- Shi YP, Sayed U, Qari SH, Roberts JM, Udhayakumar V, et al. (2004) Natural immune response to the C-terminal 19-kilodalton domain of Plasmodium falciparum merozoite surface protein Journal of Tropical Medical Hygiene 64: 2716–2723.
- Staalsoe T, Megnekou R, Fievet N, Ricke CH, Zornig HD, et al. (2009) Acquisition and decay of antibodies to pregnancy-associated variant antigens on the surface of Plasmodium falciparum-infected erythrocytes that protect against placental parasitemia. J Infect Dis 184: 618-626. Link: https://bit.ly/2VFWAcx
- Steketee RW, Nahlen BL, Parise ME, Menendez C (2001) The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg 64: 28-35. Link: https://bit.ly/3CrKhkJ
- Sule-Odu AO, Ogunledun A, Olatunji AO (2012) Impact of asymptomatic malaria parasitaemia at parturition on perinatal outcome. J Obstet Gynaecol 44: 55-59.
- Takem EN, D'Alessandro U (2013) Malaria in pregnancy. Mediterr J Hematol Infect Dis 5: e2013010. Link: https://bit.ly/3jEXz4C
- Teplin SW, Burchinal M, Johnson-Martin N, Humphrey R, Kraybill E (1991) Neurodevelopmental, health and growth status at age 6 years of children with birth weights less than 1001 grams. Journal of Pediatrics 118: 768-777. Link: https://bit.ly/3fL0Mi6
- Ter Kuile FO, Terlouw DJ, Phillips-Howard PA, Hawley WA, Nahlen BL, et al. (2003) Reduction of malaria during pregnancy by permethrin-treated bed nets in an area of intense perennial malaria transmission in western Kenya. Am J Trop Med Hyg 68: 50-60. Link: https://bit.ly/3fJrTdx
- Tyagi P, Roy A, Malhotra MS (2005) Knowledge, awareness and practices towards malaria in communities of rural, semi-rural and bordering areas of east Delhi (India). J Vect Borne Dis 42: 30-35. Link: https://bit.ly/3jAw1NW
- Verhoeff FH, Le Cessie S, Kalanda BF, Kazembe PN, Broadhead RL, et al. (2014) Post-neonatal infant mortality in Malawi: the importance of maternal health. Ann Trop Paediatr 24: 161-169. Link: https://bit.ly/2U3cVHv
- Walraven GEL, Dolmans MV (2011) The aetiology of low birthweight in a rural area of Tanzania. Tropical Medicine 28: 558-567.
- World Health Organization (2010) Insecticide-treated mosquito nets: a WHO position statement. Geneva: WHO.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
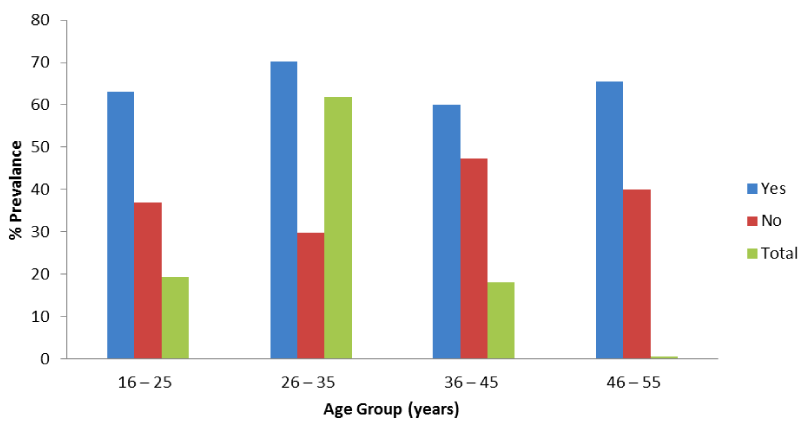




 Save to Mendeley
Save to Mendeley
