Archives of Community Medicine and Public Health
In silico and in vitro investigations of antihelmintic activities of selected approved drugs
Christopher Arinze Agube1*, Daniel Lotanna Ajaghaku2 and Ikemefuna Chijioke Uzochukwu1
2Department of Pharmacology, Faculty of Pharmaceutical Sciences, Enugu State University of Science and Technology, Enugu State Nigeria
Cite this as
Agube CA, Ajaghaku DL, Uzochukwu IC (2020) In silico and in vitro investigations of antihelmintic activities of selected approved drugs. Arch Community Med Public Health 6(2): 261-269. DOI: 10.17352/2455-5479.000118Objective: We investigated the binding affinities of some approved drugs to ascaris suum Mitochondrial Rhodoquinol Fumarate Reductase (MRFR), an essential enzyme for ascaris survival, and the possibility of repurposing these drugs as antihelmintic agents using In silico molecular docking and in vitro paralysis and mortality times of fifteen selected front runners.
Method: Two hundred approved drugs were selected from ZINC® database based on bioactivity scores while MRFR (PDB code, 3vra) was obtained from the Protein Data Bank (PDB). Both were prepared using AutoDock tools v.1.5.6 and Chimera v.1.9.The docking protocol was validated by computationally reproducing the binding of atpenin to MRFR. The selected approved drugs and the receptor were docked using AutoDockVina v. 4.0. The docking results were analyzed using PyMoL v. 1.4.1.The paralysis and mortality times of the identified frontrunners against Pheretima posthuma were determined in vitro and synergistic testings were done by the checkerboard method.
Result: Fifteen drugs had binding free energies between -7.825 to -11.025 kcal/mol while four of these drugs (mefloquine, doxycycline, mepacrine and proguanil) emerged as major frontrunners by both In silico and in vitro assessments. The paralysis and mortality times of the four drugs were between 0.33-0.50 hr as against 1.80- 2.36 hr for albendazole. They were therefore predicted to have ability to affect MRFR in the same manner as atpenin hence, suggestive of potential antihelmintic activity.
Conclusion: The antihelmintic potentials of mefloquine, doxycycline, proguanil and mepacrine have been demonstrated. In vivo investigation of these frontrunner drugs is strongly recommended.
Introduction
The fortuitous nature, enormous cost and huge time invested in traditional drug discovery and development is a major factor that has fueled the inertia in most pharmaceutical companies to engage in research into new chemical entities. This is a consequence of the unpredictability of these researches hence results often do not justify the effort. This scenario has mostly affected the neglected tropical and orphan disease domains. It is so due to the limited number of sufferers and the demography that stratifies them to the resource-poor nations [1]. As a fall out of this, pharmaceutical majors located in regions with the technology and wherewithal do not find it attractive to direct research into these areas due to marginal profit prospects.
Helminthiasis, a major Neglected Tropical Disease (NTD), has long been identified by the World Health Organization (WHO) as a disease with very high morbidity rate and cognitive deficit especially in school-aged children [2,3]. The prevalence is mostly confined to the tropical and subtropical regions where there still exists infrastructural deficit; poor sanitation; use of untreated fecal matter as fertilizers and subsisting bare soil defecation [4]. In addition, no new antihelmintic agents have been added to the list in the past decade with gradual development of resistance to the standard treatments being reported [2,5].
Drug repurposing has recorded major milestones in several treatment landscapes hence this technique has been considered a veritable tool to achieve this objective in antihelmintic sphere. It is a well-known fact that observed pharmacological activity of drugs is premised on their ability to form stable complexes with their receptors while the magnitude of activity is a function of avidity of binding. The application of computational techniques in addition to in vitro tools in modern day drug design has afforded the benefits of specificity and druglikeness optimization hence mitigating cost and time investment.
Molecular docking simulations represent the classical method in computational studies and the successes recorded using this technique both in antihelmintic and other drug discovery landscapes gave impetus for the employment of this technique in this study. Prominent among such studies is the work of Uzochukwu, et al. [6] which revealed the potentials of some approved drugs as antihelmintic agents.
This study investigated the antihelmintic potential of some approved drugs using ascaris suum mitochondrial rhodoquinol fumarate reductase enzyme as target. The in vitro paralysis and mortality times of the frontrunners using the ascaris surrogate, Pheretima posthuma, were determined.
Materials and methods
Selection and preparation of receptor
Bioinformatic mining of the Protein Data Bank (PDB) was done to identify the suitable ascaris MRFR 3D structure for the study. The crystal structure of the MRFR(PDB code, 3vra) was obtained from the Research Collaboration Standard Bioinformatics (RCSB) database.
The Flavin Adenine Dinucleotide (FAD) and hememolecules present in the structure and the extra subunits were deleted using Chimera v.1.9. The rest of the structure (A to D) were saved in Mol 2 and PDB file formats and further processed using Auto Dock tools v.1.5.6 in order to create PDBQT file suitable for molecular docking stimulations.
Selection and preparation of approved drugs
The bioactivities of the reference compound Atpenin (A5) were determined using Molinspiration online tool (www.molinspiration.com). An in-house database of approved drugs was sorted and the best two bioactivity scores of the reference compound were used to query the in-house database of the approved drugs in order to select drugs with similar bioactivity scores to the reference compound. In this instance, G-Protein Coupled Receptor (GPCR) and Enzyme Inhibitor (EI) were used for selection of approved drugs. In addition, bioactivity scores in the lower range served as negative controls.
Validation of molecular docking protocol
Validation of the docking protocol was implemented by reproducing the experimental complex of the probe compound with its receptor In silico. The receptor in complex with the probe was obtained from the RCSB database [7] by the use of bioinformatic mining and prepared for docking simulation. The probe and all hetero-molecules were deleted with Chimera v.1.9 [8]; polar hydrogen, Kollman charges were deleted then the grid box sizes and grid space centre of 10Å were determined with MGL tools v.0.1.5.6 [9]. The probe coordinates were obtained from the 3D structure in ZINC® database [10] to determine the conformation of the non-complexed atpenin prior to docking with the target.
Finally, all hydrogen atoms, torsions and all rotatable bonds were allowed in their natural states. The outputs were generated in PDBQT extension. Docked conformations were visualized in PyMol v. 1.4.1 and docked poses were compared with the experimental crystal structure of the probe by superimposition of the Atpenin (A5).
Molecular docking simulations
The molecular docking stimulations of the approved drugs and the reference compound (atpenin) were implemented using Auto Dock Vina v.4.0. The search grid was dictated by the location of the atpenin binding site on the enzyme structure. The post docking analysis was done to determine the bonds and the various amino acids involved in the binding between the protein and ligands. This was implemented using PyMol v.1.4.1. And the binding free energies of the best binding conformations of the complexes were obtained and recorded.
in vitro analysis
in vitro antihelmintic evaluation was done by determination of the paralysis and mortality times of Pheretima posthuma (adult earthworm). Pheretima posthuma was chosen as surrogate due to acute unavailability of the investigated nematodes from sacrificed livestock and the amino acid sequence similarity between P. posthuma and the investigated nematodes by virtue of a protein sequence alignment query.
Worm collection and preparation
The earthworms were harvested from swampy soil in Agulu, Anambra State, Nigeria and were stabilized in the soil marsh from where they were scooped and kept under cold chain till time of investigation. The worms were identified by Mrs. Olue Annastasia of department of Parasitology and Entomology, Nnamdi Azikiwe University, Awka, Nigeria.
Drugs: Purchase and preparation
The drugs used for the bioassay were obtained from registered pharmacies in Nigeria and a few from Boots, United Kingdom. Patent holders brands were used or brands from reputable manufacturers and seven assay points were chosen between 0.078 to 5.0 mg/ml and were prepared as stock\ solutions.
Determination of paralysis and mortality times of frontrunners
The determination of paralysis and mortality times of frontrunners among the selected approved drugs was evaluated as described by Ajaiyeoba, et al. [11]. Five worms of average weight were rinsed with distilled water and placed in 20 ml solution of each drug in a standard petri dish according to the labeled concentrations.
The petri dishes were mechanically swirled to ensure the entire worm bodies were covered in the drug solution. The worms were then monitored for paralysis and mortality times and the observations were recorded. This same procedure was replicated in the synergistic testing. Albendazole served as standard reference while distilled water was negative control.
Paralysis was assessed as a situation when the worm loses muscular tone and unable to move its body except with vigorous shaking or when pricked with an object while mortality was considered when the worm does not move its body even when placed in water bath at 50ºC.
Preparation of drugs for synergistic testing
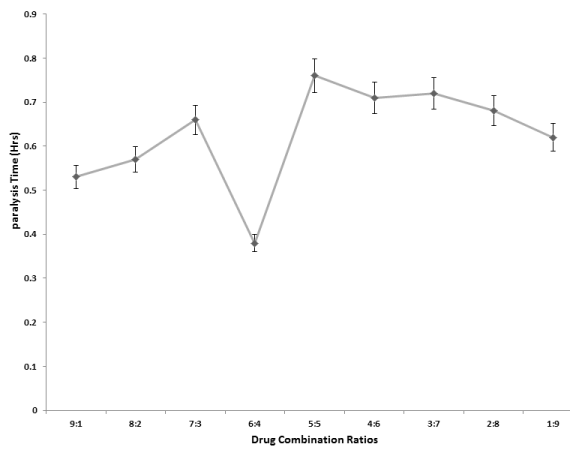
Doxycycline and mepacrine were selected for combination studies from among the four best frontrunners using effectbased strategy [12]. The combination of both drugs were prepared in 9 ratios of 5 mg/ml assay concentration (9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, 1:9). Paralysis and mortality effect of both drugs separately and when combined were used for the calculation of their combination index using the formula.
Where A = Effect of doxycycline, B = Effect of mepacrine, AB = Effect of different ratio combinations of doxycycline and mepacrine. When X = 1 (additive interaction), X > 1 (synergistic interaction) and X < 1 (antagonistic interaction).
Statistical analysis
Data collected were presented as mean ± SEM and analyzed for one-way ANOVA statistics with SPSS version 16.0. Mean differences of p <0.05 were taken to be significant.
Results
In silico antihelmintic predictions
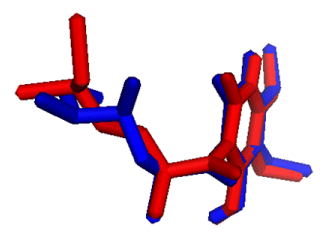
On the basis of bioactivity scores similarity of the approved drugs to atpenin, the in-house drug database was sorted and one hundred drugs were selected. And the docking protocol was validated by superimposition of the experimental atpenin A5 on the atpenin from docking which showed a near perfect fit (Figure 1).
The post docking analysis yielded two hundred drugs (including their isomers) with binding energies within the range of atpenin (–7.825 kcal/mol). Out of this list, fifteen drugs with the closest binding energies to atpenin were selected as frontrunners. This result is presented in Table 1.
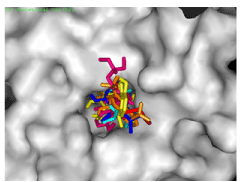
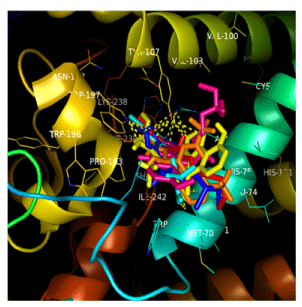
Five out of the fifteen frontrunner drugs, mefloquine (Mef), Proguanil (Prog), Doxycycline (Doxy), Albendazole (Alb), Mepacrine (Mep) and Atpenin (Atp) were found to have exploited the same binding pocket and interacted with the same amino acid residues within the receptor site (Figures 2a,2b).
Hydrogen bonding was found to have predominated among molecular interactions between the receptor and the frontrunner drugs especially amongst the polar ligands though hydrophobic interactions were also present and these hydrogen bond networks helped to strengthen the binding effect between the target and the ligands (Figures 3,4a,b,c,d).
Analogous to H-bonds are halogen bonds which are as well specific and have been found to play a major role in establishment of strong anchor points between different subunits during ligand-protein binding which leads to enhanced drug efficacy. The generous amount of fluorine atoms in the molecular structure of mefloquine resulted in high halogen bond formation with consequent optimization of the bonding of mefloquine to the receptor (Figure 5).
in vitro antihelmintic activity
The in vitro antihelmintic evaluation of the frontrunners across the seven assay points was dose-dependent and the activities of the frontrunners were significantly different from the reference standards at p<0.05 across all assay points.
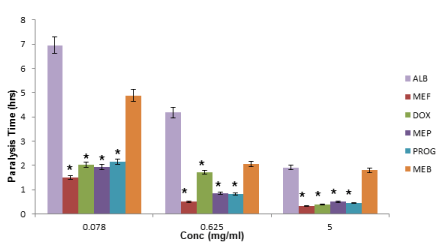
Four drugs showed the best activity in this respective decreasing order: mefloquine, doxycycline, proguanil and mepacrine. The asterisk sign represents the gradation of activity hence triple asterisk represents the highest activity while the double and single asterisk represent the next in ranking order. This paralysis and mortality time is presented in Tables 2,3. At a significance level of *P<0.05 the in vitro activity (paralysis and mortality times) of the four frontrunners were found to be far better when compared to that of the positive controls (benzoimidazoles) across three assay points (Figures 6,7).
Higher in vitro activities of the frontrunners against benzoimidazoles is congruent with the In silico observations and these were dose-dependent with the highest activity recorded at the 5 mg/ml concentration (Tables 2,3). There was observed significant difference in the mean paralysis and mortality times of the other selected approved drugs at p<0.05 using the one-way Anova statistics when compared to the means of the frontrunners with mefloquine and doxycycline respectively producing about six-fold activity as against the positive control, albendazole.
However, proguanil and mepacrine produced a four-fold in vitro activity against albendazole though at lower concentrations such as 0.078 mg/ml the mean paralysis and mortality times for doxycycline, mepacrine and proguanil were still significantly different from other drugs but the activities dropped to a double-fold.
Drug combination interactive effect
The synergistic testing produced the greatest activity (optimal fixed dose) at the 6:4 ratio of doxycycline and mepacrine (Figures 8,9). This combination produced supra-additive effect (synergism) with paralysis combination index of 2.35 and mortality combination index of 2.23.
Discussion
The choice of the 3vra receptor out of the four ascaris protein structures deposited in PDB was informed by the fact it is the only that is bound to an experimental compound which offered a guide as to the wet laboratory binding conformation and orientation while the successful superimposition of the atpenin experimental structure on the x ray crystallographic structure validated the docking protocol that was implemented.
When drugs bind to their receptors the conformation with the lowest binding energy is considered the most energetically favourable spatial arrangement and thus is speculated to have better binding affinity. An analysis of the binding free energies of the selected drugs docked with the ascaris MRFR showed the binding free energy of atpenin to be -7.825 kcal / mol which was taken as the benchmark value for assessment of the antihelminthic activity for the drugs investigated.
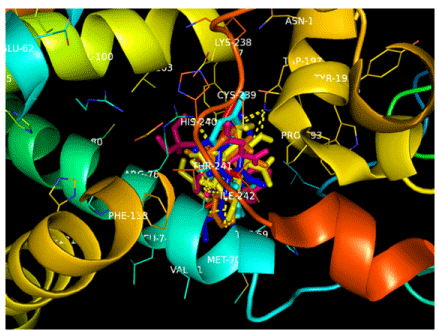
Seventy compounds produced binding energies far less than that of the reference compound, atpenin and fifteen among these were chosen as frontrunners which included mefloquine, proguanil, mepacrine, doxycycline, chloroquine, mebendazole and albendazole. The ligands were observed to have exploited the same binding pocket and interacted with same amino acid at the active site and these observations are suggestive of these drugs having the ability to affect the target enzyme system in the same way as atpenin which is indicative of potential antihelmintic activity. Molecular interactions were made with Serine 72, Histidine75, Methionine 70, Tyrosine 107, Arginine 76, Tryptophan 197, Valine 71, Isoleucine 242, Phenyalanine 138, Lysine 238, Proline 193 and Cysteine 239 and these similar interactions predict similar biological activity.
Patil, et al. [3] postulated that weak intermolecular interactions play major role in energetically stabilizing ligands at the binding site of proteins and which was corroborated by some other works. These intermolecular forces, they described as hydrophobic interactions, Van der Waals forces and hydrogen bonds (H-bonds). And further went on to demonstrate that hydrogen bonds facilitate molecular interactions and enhance receptor-ligand interactions in situations where donor and acceptor possess stronger or weaker hydrogen and oxygen than the surrounding water.
The role of hydrogen bonds in modulating receptor-ligand binding affinity is well recognized and documented which is usually a result of setting up of like- for- like synergistic interactions and Chen, et al. [13] submitted that this is usually due to displacement of protein-bound water to the surrounding bulk water since in biological systems there exists consistent competition between hydrogen bond and surrounding bulk water. They substantiated this in a study that employed hexadecane partition coefficient and reported a synergistic receptor-ligand hydrogen bond pairings that potentiated high affinity binding through elimination of the interference usually associated with bulk water. H-bond plays a very critical role in binding ligands and aids in expression or suppression of activity [14].
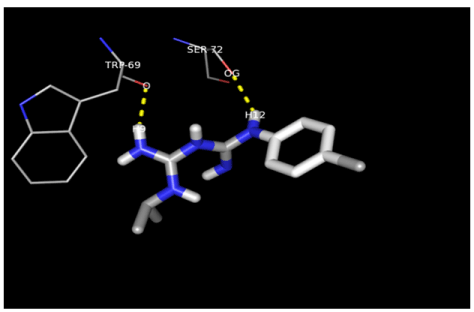
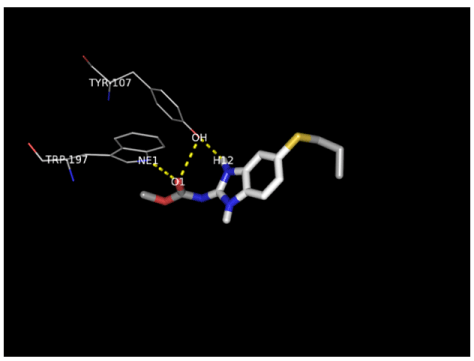
H-bonding was prominent in the interactions between ligands and the receptor in this study and of special note are the interactions between doxycycline, proguanil, atpenin and albendazole and the protein which involved the NE1 atom of Trp197 and O5 as well as OH of Tyr107 and O7 atoms. Ser72 also made polar contacts with OG and O2 atoms while albendazole formed H-bonds with Tyr107 OH group and the H12 atom. Polar contacts were also made at NE1, O1 and H12 atoms with Tyr197.
There was observed relative flexibility of the hydroxyl groups in Tyr107, Trp197 and Ser 72 that generated free energy change which enhanced the hydrogen bond interactions with the hydroxyl groups leading to the s-s pairing of H-bonds between the carboxyl and amine groups of albendazole, atpenin and doxycycline. Additional H-bonds were formed by the interaction of the guanidinium groups of arginine with doxycycline because of increased number of proton donors which is expected to affect its pharmacokinetic properties in vivo.
However, Verma, et al. [15] averred that optimization of ligand affinity could also be achieved through incorporation of hydrophobic atoms at the site of hydrogen bonding thereby compromising the effect of hydrogen bonding and this according to Qian, et al. [16] would improve significantly the biological activity of such drugs. The effectiveness of this strategy was demonstrated by Patil, et al. [3] using 4-amino substituents that possess mainly hydrophobic atoms against c-Src and c-Abl kinases to optimize the inhibitory activity of the ligand.
Mefloquine and mepacrine made hydrophobic interactions which may be viewed as entirely driven by desolvation. This is because a hydrophobic ligand (or surface on the binding site) usually disrupts the structure of bulk water and decreases entropy due to stronger bonding and ordering of water molecules around the solute [17]. These hydrophobic bonding were nonspecific but strong and the hydrophobic effect correlated with the partition between aqueous and non-polar solvents. The interactions of the hydrophobic ligands were mostly with the hydrophobic residues Isoleucine, Phenyalanine, Methionine. The importance of hydrophobic interactions in protein-ligand binding cannot be overemphasized as it enables optimization of lead compounds.
The contribution of halogen-bonding in protein-ligand binding is currently receiving deserved attention and acknowledgement. According to Patil, et al. [3] There is remarkable increase in biological activity of compounds bearing halogen substituents following conformational changes during binding and Lu, et al. [18] further affirmed that of all the halogens the binding affinity associated with fluorine is markedly higher. The importance of halogen bond in ligand optimization is underscored by the fact that its net free energy is not affected by degree of desolvation in contrast to H-bonds. This observation was made in our study as mefloquine with six fluorine atoms in its structure produced the least binding free energy (high binding affinity} among the frontrunner drugs which enhanced its biological activity thus confirming the positive and desirable effect of halogen bonding in protein-ligand binding.
Uzochukwu, et al. [6] reported similar results while targeting same fumarate reductase enzyme with some approved drugs and interestingly their research produced many antimalarials with good antihelmintic activities as was the case in this study. Among these antimalarials were mefloquine, proguanil, quinine, chloroquine, pyrimethamine and mepacrine. There is evidence of cross activity between antimalarials and antihelmintics for which reason the employment of oil of chenopodium and santonin both in malarial and helminth chemotherapy is speculated to stem from an identical mode of action of the two drug classes wherein they form heme complexes in parasite food vacuoles obstructing glucose uptake [19]. Mepacrine and chloroquine share structure similarity and have for years been employed as treatments for teniasis and N. americanus infections hence further strengthening the cross activity theory [20].
The action of antihelmintics could be by any of disruption of parasite metabolism or destruction of cuticle/cytoskeleton which eventually leads to paralysis and eventual death [21]. Ekeanyanwu and Etienjirhevwe [22] reported the interference with energy generation in helminth parasites by phenolic compounds and postulated that the mechanism of action seemed to be connected to the uncoupling of specific reductase- mediated reactions. Coincidentally, the target in this study, also a reductase, is an essential enzyme for adult helminth anaerobic metabolism where it catalyzes conversion of fumarate to succinate therefore ability of a chemical entity to block the activity of this enzyme would ultimately lead to parasite mortality hence predicting significant antihelmintic capacity. This novel mode of action of these approved drugs could hopefully address the developing parasite resistance to the current human antihelmintic agents. The works of Adeniran and Sonibare [23] corroborated this mode of action that is associated with phenolic compounds when extracts of Dioscorea bulbifera, Mondora myristica and Xylopia aethiopia were found to interfere with oxidative phosphorylation reactions.
Roy [24] reported a central nervous system action of some alkaloids on earthworms for which reason it was speculated that mepacrine, an alkaloid could possibly have yet another mechanism of action. Raghavanma and Rama [25] also reported a dose-dependent antihelmintic activity of different extracts of Nauclea orientalis leaves which they opined to be a consequence of their tannin and saponin content. Notwithstanding that the antihelmintic activities observed in the present study compared and correlated very well with most of these studies, our results were viewed as superior since they were obtained at much lower assay points thus presupposing higher activities at higher concentration. This therefore gives hope for development of more effective and tolerable chemotherapy as drug safety is optimized when effective at lower concentrations.
The activities of drugs have been shown to be affected by interaction with other drugs which could be beneficial or adverse. Certain pharmacokinetic factors such as solubility, bioavailability, metabolism (which can result from enzyme induction or inhibition) do impact on the activity of a drug [26]. Many drug preparations have provided better therapeutic benefits through this process and combination therapies are fast becoming a norm in most therapeutic landscapes and in the opinion of Portsmouth [27], “drug combinations are considered the ideal therapy for treatment of important infectious diseases including HIV, malaria and TB”. Hu, et al. [28] noted that of all the antihelmintics currently in use, only tribendimidine (a nicotinic acetycholine receptor agonist (nAchR) and albendazole – a benzoimidazole are adequate for single dose mass drug administration. This in their opinion is because both have excellent activity against ascaris, moderate against hookworms and poor activity against threadworm (Strongyloides) and whipworm. But a major drawback however is that tribendimidine is still under trial and unapproved except in China hence, raises urgent need to explore combination therapies against helmintic infections.
The recorded supra-additive interaction (synergism) between doxycycline and mepacrine may be as a result of the documented inhibition of the metabolism of mepacrine by doxycycline and this is of great interest given the inadequacies of single molecule therapy of antihelmintics currently in addition to the increasing wave of resistance to available treatments. The works of Keiser, et al. [4] confirmed these inadequacies and the effectiveness as well as the desirability of combination therapy in antihelmintic chemotherapy. It is therefore hoped that this strong positive interaction observed with the combination would be replicated in clinical studies and lead to the full repurposing of these two approved drugs as new antihelmintic agents.
Sincere appreciation goes to Dr Ezebuo Fortunatus for the assistance provided during the study and the authors state that there are no conflicts of interest in this study.
- WHO (2012) Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases: a Roadmap for Implementation. Geneva: World Health Organization. Link: https://bit.ly/3fAujtH
- Prichard RK (2007) Ivermectin Resistance and Overview of the Consortium for Anthelmintic Resistance SNPs. Expert Opin Drug Discov 2: S41-S52. Link: https://bit.ly/2J9pE5H
- Patil R, Das S, Stanley A, Yadav L, Sudhakar A, et al. (2010) Optimized Hydrophobic Interactions and Hydrogen Bonding at the Target-Ligand Interface Leads the Pathways of Drug-Designing. PLoS ONE 5: e12029. Link: https://bit.ly/3fDwf4s
- Keiser J, Tritten L, Adelfio R, Vargas M (2012) Effect of combinations of marketed human anthelmintic drugs against Trichuris murisin vitro and in vivo. Parasit Vectors 5: 292. Link: https://bit.ly/368M1RV
- Smout MJ, Kotze AC, McCarthy JS, Loukas A (2010) A Novel High Throughput Assay for Anthelmintic Drug Screening and Resistance Diagnosis by Real-Time Monitoring of Parasite Motility. PLoS Negl Trop Dis 4: e885. Link: https://bit.ly/368L5Nd
- Uzochukwu IC, Olubiyi OO, Akpotor CO (2014) Determination of Binding Affinities of some Approved Drugs to ascaris suum Mitochondrial Rhodoquinol Fumarate Reductase by In silico Molecular Docking. J Pharm All Sci 11: 2114-2124. Link: https://bit.ly/39jGcTA
- Bissantz C, Kuhn B, Stahl M (2010) A medicinal chemist’s guide to molecular interactions. J Med Chem 53: 5061-5084. Link: https://bit.ly/2HDjpXm
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, et al. (2004) UCSF Chimera–a Visualization System for Exploratory Research and Analysis. J Comput Chem 25: 1605–1612. Link: https://bit.ly/3maUZDN
- Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, et al. (1998) Automated Docking using a Lamarckian Genetic Algorithm and an Empirical Binding Free Energy Function. J Comput Chem 19: 1639-1662. Link: https://bit.ly/3l8jkbS
- Irwin JJ, Sterling T, Mysinger MM, Bolstad ES, Coleman RG (2012) ZINC: A Free Tool to discover Chemistry for Biology. J Chem Inf Model 52: 1757-1768. Link: https://bit.ly/3maTg15
- Ajaiyeoba EO, Onocha PA, Olarenwaju OT (2001) in vitro Anthelmintic Properties of Buchholzia coriacease and Gynandropsis gynandra extract. Pharm Biol 39: 217-220. Link: https://bit.ly/2V6yKTC
- Foucquier J, Guedj M (2015) Analysis of drug combinations: current methodological landscape. Pharmacol Res Perspect 3: e00149. Link: https://bit.ly/2JcMXvA
- Chen D, Oezguen N, Urvil P, Ferguson C, Dann SM, et al. (2016) Regulation of protein-binding affinity by hydrogen bond pairing. Sci Adv 2: e1501240. Link: https://bit.ly/2JjZVHC
- Wade RC, Goodford PJ (1993) Further development of hydrogen bond functions for use in determining energetically favorable binding sites on molecules of known structure. II. Ligand probe groups with the ability to form more than two hydrogen bonds. J Med Chem 36:148-156. Link: https://bit.ly/3nXqMIB
- Verma S, Joshi KB, Ghosh S (2007) Peptide-based soft materials as potential drug delivery vehicles. Med Chem 3: 605-611. Link: https://bit.ly/3mm13ZW
- Qian SB, Waldron L, Choudhary N, Klevit RE, Chazin WJ, et al. (2009) Engineering a ubiquitin ligase reveals conformational flexibility required for ubiquitin transfer. J Biol Chem 284: 26797-26802. Link: https://bit.ly/3l8pLvH
- Maurer M, Oostenbrink C (2019) Water in protein hydration and ligand recognition. J Mol Recognit 32: e2810. Link: https://bit.ly/33fSRmE
- Lu Y, Wang Y, Xu Z, Yan X, Luo X, et al. (2009) C-X…H contacts in biomolecular systems: how they contribute to protein-ligand binding affinity. J Phys Chem B 113: 12615-12621. Link: https://bit.ly/3lhKX2x
- Frayha GJ, Smyth JD, Gobert JG, Savel J (2002) The mechanism of action of antiprotozoal and antihelmintic drugs in man. Gen Pharmacol 28: 273-299. Link: https://bit.ly/2HIdDE2
- Rajbir S (2010) Synthetic Drugs. Mittal Publicaition 191-206.
- Nikesh M, Binitha G, Rekha S, Ravindra N, Anto-Sharing M (2011) Comparative in vitro Anthelmintic Activity of Chloroform and Acetone Extracts of menthe piperita. Int J Pharm Biol Arch 2: 945-948. Link: https://bit.ly/2Vbsmu1
- Ekeanyanwu RC, Etienjirhevwe OF (2012) in vitro anthelmintic potentials of Xylopia aethiopica and Mondora myristica from Nigeria. Afr J Biochem Res 6: 115-120. Link: https://bit.ly/2V7oRVw
- Adeniran AA, Sonibare MA (2013) in vitro potential anthelmintic activity of bulbils of Dioscorea bulbifera L. on earthworms and liverflukes. J Pharmacognosy Phytother 5: 196–203. Link: https://bit.ly/3md69b1
- Roy H, Chakraborty A, Bhanja S, Nayak BS (2010) Preliminary phytochemical investigation and anthelmintic activity of Acanthospermum hispidum DC. J Pharm Sci Technol 2: 217-221. Link: https://bit.ly/33huHIk
- Raghavanma STV, Rao NR (2010) in vitro evaluation of Anthelmintic Activity of Nauclea orientalis leaves. Indian J Pharm Sci 72: 520-521. Link: https://bit.ly/369Nt6t
- Barry M, Feely J (1990) Enzyme Induction and inhibition. Pharmacol Ther 48: 71-91. Link: https://bit.ly/2V6W97s
- Portsmouth S, Stebbing J, Gazzard B (2003) Current treatment of HIV infection. Curr Top Med Chem 3: 1458-1466.
- Hu Y, Xiao SH, Aroian RV (2009) The new anthelmintic tribendimidine is an L-type (levamisole and pyrantel) nicotinic acetylcholine receptor agonist. PLoS Negl Trop Dis 3: e499. Link: https://bit.ly/36aJjLL
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.






 Save to Mendeley
Save to Mendeley
