Archives of Community Medicine and Public Health
Immunological outcomes between Tenofovir versus Zidovudine-based regimens: a retrospective cohort study
Meshack Lugoba1, Manase Kilonzi1, George M Bwire2*, Pacifique Ndayishimiye1, Wigilya P Mikomangwa1, Hamu J Mlyuka1, Alphonce I Marealle1, Ritah F Mutagonda1 and Kennedy D Mwambete2
2Department of Pharmaceutical Microbiology, School of Pharmacy, Muhimbili University of Health and Allied Sciences, Box 65001, Dar es Salaam, Tanzania
Cite this as
Lugoba M, Kilonzi M, Bwire GM, Ndayishimiye P, Mikomangwa WP, et al. (2019) Immunological outcomes between Tenofovir versus Zidovudine-based regimens: a retrospective cohort study. Arch Community Med Public Health 5(2): 043-048. DOI: 10.17352/2455-5479.000052Background: Since 2013, Tenofovir (TDF) and Zidovudine (AZT) based regimen are alternatively used as a first line for treatment of HIV in Tanzania. CD4+ cells count, which is recommended after every six months monitors the immunological progression, and used as one of the indicators of treatment progression. However, there is little literature available information to compare the immunological outcomes of these two regimens.
Methods: A hospital-based retrospective cohort study was conducted at Muhimbili National Hospital, Care and Treatment Clinic (MNH-CTC) in Dar es Salaam. HIV/AIDS patients’ files of October 2012 through November 2016 were reviewed. Using statistical package for social sciences (SPSS version 23), the mean CD4+ cells count were compared by independent t-test while Chi-square test was performed to associate categorical variables. Binary logistic regression was used to control variables such as sex and body mass index (BMI). The results were considered having statistical significance when the P < 0.05.
Results: Overall 340 patients’ files were eligible and reviewed. Of 340 patients, 50% (170) patients were on TDF. Most of the patients on TDF (64.4%) and AZT (62.5%) were female where 69.4% on TDF and 65.3% on AZT were at the age group between 25-45 years. Majority of patients 94% and 89% on TDF and AZT respectively had BMI ≥ 18.5 kg/m2.
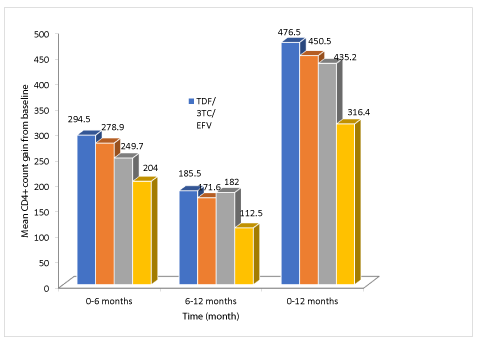
Most of the patients on TDF 54% (170) had a baseline CD4+ counts between 250-600 cells/mm3 and AZT 58% (170) had CD4+ counts <250 cells/mm3. In 12 months post antiretroviral (ARV) initiation, the mean (±SD) CD4+ counts gain in TDF regimen was higher 455.8(165.8) than that in AZT regimen 383.4 (90.6) (p<0.001). Tenofovir/Lamivudine/Efavirenz (TDF/3TC/EFV) recorded the highest mean gain [standard deviation (±SD)] CD4+ counts of 476.5(190.3) followed by Zidovudine/Lamivudine/Nevirapine (AZT/3TC/NVP);435.2 (134.9), TDF/3TC/NVP; 450.5 (201.9) and AZT/3TC/EFV; 316.4 (152.4).
Conclusion: Twelve months post ARV initiation; TDF based regimens had good immunological outcomes compared to AZT based regimens. Generally, TDF/3TC/EFV is the regimen that demonstrated highest mean CD4+ counts while AZT/3TC/EFV recorded the lowest mean CD4+, cells count.
Abbreviations
3TC: Lamivudine; AIDS: Acquired Immune Deficiency Syndrome; AZT: Zidovudine; BMI: Body Mass Index; CTC: Care and Treatment Clinic; EFV: Efavirenz; HIV: Human Immunodeficiency Virus; MNH: Muhimbili National Hospital; NNRTIs: Non-nucleoside Reverse Transcriptase Inhibitors; NRTIs: Non-Nucleoside Reverse Transcriptase Inhibitors; NVP: Nevirapine; SD: Standard Deviation; TDF: Tenofovir; WHO: World Health Organization; IPT: Intermittent Preventive Therapy; CPT: Co-Trimoxazole Preventive Therapy; IPT: Intermittent Preventive Therapy; CRF: Case Report Form; PLHIV: People Living with HIV
Introduction
Acquired Immune Deficiency Syndrome (AIDS) is still a burden in sub-Saharan African countries. The world health organization (WHO) estimates 36.7 million people live with HIV (PLHIV) whereby 1.8 million people are newly infected and 1.0 million die globally [1,2]. Sub-Saharan Africa is the most severely affected region and contributes about two third of the total number of people living with HIV in the world [3].
In Tanzania 2016-2017, approximately1.4 million people live with HIV, with prevalence of 5% among adults (15 to 64years). It is reported that approximately 81,000 new cases of HIV occur among adults and 33,000 AIDS-related deaths are reported annually [4]. PLHIV in Tanzania aged 15 to 64 years who know their HIV status, 90.9% self-report current use of ART [4].
A person gets infected with HIV either by sexual intercourse, sharing of sharp equipment, vertical transmission (from mother to child), blood transfusion, organ transplants or occupational accidents, especially for healthcare workers [5]. In the body of an infected individual HIV are mainly found in the blood, sexual fluid and breast milk.
HIV once in the body depends on CD4+ cells for its survival and replications [6]. CD4+ cells (also called T helper cells) play a great role in coordinating the immune system of an individual. HIV attacks and destroys immune systems of the human body. The destruction of these cells by viruses manifest as immunodeficiency syndrome [6]. Individuals infected with HIV experience a range of infections from other micro- organism generally are called opportunistic infections [5,6]. The introduction of antiretroviral drugs (ART) in 1987 has helped to prolong the lives of those people who are infected with HIV and in prevention of HIV transmission [7].
Different regimen had been introduced and removed from the market due to intolerable side effects, affordability and availability. In 2006, WHO recommended changing from stavudine-based regimen because of toxicity and Zidovudine/Tenofovir (AZT/TDF) based combination were the alternative regimens [8]. By 2010 majority of the countries already moved to AZT/TDF based combinations [9] and AZT based regimens in particular were approved to be the first line in Tanzania.
Due to the advantages of TDF over other nucleoside reverse transcriptase inhibitors (NRTIs); high tolerability, associated with good adherence (available as a once-daily fixed-dose combination), active against hepatitis B, robust to acquisition of resistance associated mutation, safe during pregnancy and in children above 2 years of age [10], hence TDF is more preferred over AZT. In 2015 Tanzania, prequalified TDF based regimen as the first line regimen in prolonging the life of people who are living with HIV (PLHIV) including pregnant women [11].
Despite the several advantages, TDF has some shortcoming including its association with kidney toxicity and bone density loss, hence AZT based regimens are used as alternative regimens [12]. The main aim of ART is to lower the viral load and improve the immune function. Successful treatment by ART is associated with depression of viral load and a significant gain in CD+ T cell count. Monitoring clinical signs and symptoms of the disease with laboratory counting of CD+ T cells and viral load is useful in monitoring the disease progression and response of patients to the regimen [13].
Since the introduction of TDF-based regimen in Tanzania, there is limited information on the difference in CD4+ count gaining between TDF-based regimens and AZT based regimens. Therefore, this study aimed to compare the CD4+ T cell changes as the measure of immunological outcomes among HIV/AIDS patients using TDF versus AZT based regimen.
Methods
Study design, population and area
This was a retrospective study conducted on patients above 14 years, who were under TDF and AZT based regimens. The study was conducted at Muhimbili National Hospital (MNH) which is a national referral hospital located in Dar-es-Salaam, Tanzania. MNH has 1500 bed facility, attends 1,000 to 1,200 out patients in outpatient department (OPD) per day and admits 1,000 to 1,200 inpatients per day. The hospital has separate CTC offering services to more than 5,000 patients (PLHIV), about 500 patients per week.
Study period
The study was conducted from February to April 2018, whereby patients’ information from October 2012 to November 2016 were extracted using a case report form (CRF).
Sample size and sampling technique
The sample size of 340 was used in this study, the sample was obtained by using mean gain in CD4+ and a standard deviation obtained from a previous study conducted in Ethiopia [14]. A ratio of 1:1 was applied whereby 170 patients on AZT and 170 patients on TDF were recruited. Additionally, a ratio of 1:1 was maintained within AZT and TDF group, whereby 85 patients on Efavirenz and 85 patients on Nevirapine were recruited. Patient file records were selected by using systematic random sampling techniques.
Inclusion and exclusion criteria
Patients aged ≥ 14 years old, on TDF or AZT based regimen for at least 6 months, with baseline and 6 months CD4 counts, were involved in this study. Patients whose ART regimen was changed in less than 6 months after the initiation of therapy and pregnant women were excluded from the study.
Data collection
The CRF that consisted of two parts was used in data collection. The CRF was validated before the commencement of the study. The first part of the CRF consisted of the demographic information such as sex, age, weight, height and marital status. Body mass index (BMI) was calculated by dividing weight (kg) over height (m). The second part of the CRF consisted of information regarding the baseline WHO clinical stage (before initiation of the therapy), type of regimen initiated, and baseline, and subsequent CD4+ counts.
Data management and analysis
Data from the CRF were entered in the excel sheet then transferred to statistical package for social sciences (SPSS software version 23 Chicago Inc., USA) for cleaning and analysis. Bar charts and tables were utilized during data summarization accordingly. Independent t-test was used to compare the mea`n CD4+ counts within and between the regimens. Chi-square test was performed to determine association between immunological changes and demographic variables. Where logistic regression was used to identify the additional predictors of CD4+ recovery and give the strength of association. Odd ratio with 95% confidence interval was used as a measure of strength of association and the results were considered having statistical significance when the P < 0.05.
Result
Socio-demographic characteristics of the study participants
A total of 340 patients’ files were eligible and reviewed. Of 170 patients on TDF 64.4% were females and for those on AZT 62.5% among 170 patients were also females. Most of the participants 69.4% on TDF and 65.3% on AZT were within the age group between 25-45 years. Majority of the patients had BMI ≥ 18.5 kg/m2 where 93.5% on TDF and 88.8% on AZT regimen. Majority of the patients were married, 42.3% on TDF and 40% on AZT group (Table 1).
Baseline clinical characteristics of the study participants
Most of the study participants in both regimens were in WHO clinical stage 2 and 3 prior to initiation of therapy. Majority of patients on TDF group (53.5%) had baseline CD4 counts between 250-600 cells/mm and (57.6%) on AZT group had baseline CD4 counts of below 250cells/mm3. Fifteen (8.8%) patients on TDF and 12 (7.1%) on AZT had encountered TB infection and were treated during the period of follow-up. The findings show that, only 43 (25.3%) patients on TDF and 12 (7.1%) on AZT, received isoniazid preventive therapy (IPT) and 11 (6.5%) patients on TDF and 14 (8.2%) on AZT were using cotrimoxazole (CTX) as prophylaxis of opportunistic infections during the time of follow-up (Table 2).
Immunological changes between regimens over time
Over the study period, the mean (SD) CD4+ counts gaining were more observed in TDF based regimens over AZT based regimens; the mean (SD) CD4+ count gained in6 months from the baseline in TDF group was 272.1 ± 118.4 and in AZT group 241.5 ± 122.6. The mean CD4+ count gaining difference of +59.130 was observed between the two groups (p=0.005). In 12 months, the mean (SD) CD4+ count gained from the baseline in TDF group was 455.8± 165.8 while in AZT group it was 383.4 ± 190.6. The mean CD4+ count gaining difference of +100.85 was observed (p<0.001). The increase in CD4+ count was rapid during the early months of using ART but the CD4+ gain decreased as the patients stabilized on the treatment (Tables 3,4).
Immunological changes within TDF and AZT based regimens
The higher mean CD4+ count gaining was observed in TDF/3TC/EFV regimen compared to TDF/3TC/NVP but the difference was not statistically significant thru out the follow-up period of treatment. The AZT/3TC/NVP regimen had significant mean CD4+ counts gaining compared to the AZT/3TC/EFV, whereby at 6 months from baseline was 278.9 ± 110.4 and 204.0 ± 123.3 of CD4+ and 12 months was 450.5 ± 201.9 and 316.4 ± 152.4 respectively (Tables 3,4 and Figure 1).
Predictors of CD4+ count gaining
On binary logistic regression sex, age, BMI, and WHO clinical stages showed significant association with mean CD4+ counts gaining at 12 months of using ART, whereby male were 2 times (OR-1.992, P- value = 0.003, 95% CI= 1.266 – 3.134) more likely to have mean CD4+ counts of < 600 cells/mm3 compared to female. Those aged > 45 years old were 10 times (OR- 10.185, P- value < 0.001, 95% CI = 2.832 – 36.636) more likely to have mean CD4+ counts of < 600 cells/mm3, those with body mass index (BMI) of < 18.5 kg/m2 are about 5 times (OR- 4.794, P- value < 0.001, 95% CI = 2.066 – 11.123) more likely to have mean CD4+ counts of < 600 cells/mm and on WHO clinical stage, those in stage 4 were 13 times (OR- 13.214, P- value < 0.001, 95% CI = 3.216 – 54.298) likely to have mean CD4+ counts of < 600 cells/mm3.
Discussion
HIV/AIDS treatment outcome depends on consistent use of ART and type of ART regimen used. Viral load and CD4+ counts changes are used as predictors of virological and immunological outcomes [14,15].
In this study, significant increase in mean CD4+ counts gaining was observed in TDF based regimens than AZT based regimens, the increase in mean CD4+ counts was rapidly observed during the first 6months of treatment, followed by a slight gain as patients were stabilized with the therapy. The same trend in CD4+ count change was observed in Ethiopian study, which reported a rapid gain in CD4+ counts 10 months after initiation of therapy and that the rate of change was the same in both regimens [15]. The difference could be due to methodological difference by which our study included all patients on either of regimen within six months while the Ethiopian study included naïve HIV patients. However, ethnicity difference of participants between these two studies could be a contributing factor as they may respond differently [16].
The gain in mean CD4+ count was more in TDF based regimen than AZT regimen which is similar to Ayele et al, 2017 in Ethiopia who reported TDF based regimens, as more efficacious compared to AZT based regimens based on mean CD4+ counts gaining. In this study TDF/3TC/EFV regimen demonstrated high mean CD4+ counts gaining followed by AZT/3TC/NVP then TDF/3TC/NVP and lastly AZT/3TC/EFV. The findings are consistent with study from Ethiopia, which found that at every 6 months of using ART, the maximum gain in mean CD4+ counts was observed in TDF/3TC/EFV followed by AZT/3TC/NVP [14]. In line with our finding, a study, which was done in Nigeria, reported that AZT/3TC/NVP has maximum immunological response compared to TDF/3TC/NVP [17].
The higher gain in CD4 for TDF than AZT regimen could be attributed by good patients’ adherence because the drug is available in once-daily fixed-dose combination (FDC) compared to AZT which is available as multiple pills or twice daily regimens, more than 95% adherence is required for better treatment outcome of HIV, however our study did not measure level of adherence among participants [18]. Moreover, TDF is more robust towards acquisition of resistance-associated mutations [10].
In this study AZT/3TC/NVP and TDF/3TC/NVP was found to have high mean CD4+ counts gaining over AZT/3TC/EFV which is in contrary to what reported by the HIV-causal collaboration in 2012, that individuals on Nevirapine regimens experienced a smaller 12-month increase in CD4+ cell count, and that they were more likely to have virologic failure at 12 months as those on Efavirenz regimens [19]. It was also reported by Nachenga et al, 2008 that the patients on Nevirapine based regimen had great risk of virologic failures, death, and regimen discontinuation and less likely to achieve good adherence compared to Efavirenz based regimen [20]. The difference observed between Nevirapine and Efavirenz based regimen in immunological response is mainly due to their pharmacokinetics and pharmacodynamics interaction between NNRTIs and NRTIs which are used in a backbone of many first line antiretroviral regimens.
Other factors which are independently associated with positive or negative mean CD4+ counts changes are age, WHO clinical stage prior to initiation of antiretroviral therapy, body mass index (BMI). Adults’ patients aged above 45 years were 10 times more likely to have mean CD4+ counts of < 600 cells/mm3 compared to young adults’ patients. This indicated that as you grow older the means CD4+ cells count tend to decrease. This result is in agreement with a study conducted in Ethiopia and Ghana, which reported that, as patients grow old; mean CD4+ counts gaining are likely to decrease [14,21]. Aging process poses great challenges to cells diversity because of thymic function deterioration. Thymic output of new cells weakening compromise diversity of T cell including CD4+ cells (T helper cells) [22].
In this study patient started ART when in WHO clinical stage 1 had the high mean CD4+ counts gaining compared to patients started ART when in WHO clinical stage 4. Having more CD4+ counts gaining implies that patients are well responding to the medications and well protected from opportunistic infections. This is in consistent with Elijah paintsil study conducted in USA reported that CD4+ T cell monitoring is more appropriate than virologic monitoring because a decreasing CD4+ T cell count is a better predictor of disease progression [23]. This finding also support WHO recommendation of diagnosis and treatment with considering CD4+ counts levels and WHO-HIV clinical stages.
Before the current WHO recommendation on HIV diagnosis and treatment, BMI was used as one of the criteria during initiation of ART. In this study, body mass index (BMI) was significantly associated with mean CD4+ counts, whereby PLHIV with BMI < 18.5 kg/m2 were 5-fold risk of having mean CD4+ counts < 600 cells/mm. These results are similar to what was reported by Langford et al, in which BMI was found to be a predictor of disease progression [24], and to what was reported in Abidjan, Ivory coast and Netherland, whereby BMI of 17 – 18 kg/m2 and < 16 kg/m2was found to correlate with 2-fold and 5-fold respectively [25,26].
Limitations
This study was limited in assessing the adherence of patients’ adherence to the ARV because in most cases this information was not captured in the patients’ files. In addition accuracy of the information solely depended on the availability of documented information in patients’ file.
Conclusion
Finding from this study demonstrate that patients on TDF based regimens have good gaining in mean CD4+ counts compared to patients on AZT based regimens. TDF/3TC/EFV express high mean CD4+ counts gaining followed by AZT/3TC/NVP. Age and baseline WHO clinical stages display independently association with gaining in mean CD+ counts among people living with HIV on TDF and AZT antiretroviral medications risk of AIDS defining stage.
Ethics approval
Ethical clearance was obtained from the Senate Research and Publications Committee of Muhimbili University of Health and Allied Sciences in Dar es Salaam, Tanzania. In addition, permission to access MMH-CTC and patients files’ records was requested from Muhimbili National Hospital Management.
We would like to thank MNH management for their support and permission to access MNH-CTC facility during the course of data collection.
- UNAIDS (2016) Global AIDS Update 2016. World Heal Organ 422. https://bit.ly/2JteEh7
- Liu AY, Buchbinder SP (2017) CROI 2017: HIV epidemic trends and advances in prevention. Top Antivir Med 25: 35–50. https://bit.ly/2JC1i18
- Joint United Nations Programme on HIV/AIDS (UNAIDS) (2015) UNAIDS World AIDS day Fact sheet 2015. UNAIDS Fact sheets 1–8.
- NBS, OCGS, ICAP, TACAIDS, ZAC, et al. (2017) Tanzania HIV Impact Survey (THIS) 2016-17: Preliminary findings 1-5. https://bit.ly/2LOFXUP
- Shubber Z, Mishra S, Vesga JF, Boily MC (2014) The HIV Modes of Transmission model: a systematic review of its findings and adherence to guidelines. J Int AIDS Soc 17: 18928. https://bit.ly/2XVaqXL
- Mishra S, Dwivedi SP, Dwivedi N, Singh RB (2009) Immune Response and Possible Causes of CD4+T-cell Depletion in Human Immunodeficiency Virus (HIV) - 1 Infection. Open Nutraceuticals J 2: 46–51. https://bit.ly/2XBcyEN
- Vella S, Schwartländer B, Sow SP, Eholie SP, Murphy RL (2012) The history of antiretroviral therapy and of its implementation in resource-limited areas of the world. AIDS 26: 1231-1241. https://bit.ly/2S7NNdj
- World Health Organization (2006) Antiretroviral Therapy for Adults and Adolescents: Recommendations for a public health approach. World Heal Organ 1-134. https://bit.ly/30xnNeb
- Duber HC, Dansereau E, Masters SH, Achan J, Burstein R, et al. (2015) Uptake of WHO recommendations for first-line antiretroviral therapy in Kenya, Uganda, and Zambia. PLoS One 10: 1-12. https://bit.ly/2LTRFNM
- Frontieres MS (2012) Rationale for Tenofovir as the First Choice in the First-Line Treatment of HIV. https://bit.ly/2YNhZNr
- Ministry of Health and Social Welfare Tanzania (2015) Tanzania National guidelines for the management of HIV and AIDS. Natl Guidel Manag HIV AIDS 1-269. https://bit.ly/2HITFEd
- Ryan J, Abdul-Basit CSS, Herrera K (2015) Considerations in first-line therapies for treatment- Naïve chronic hepatitis B 1–13. https://bit.ly/2YNYgwS
- WHO (2016) Clinical guidelines: antiretroviral therapy. 129.
- Ayele T, Jarso H, Mamo G (2017) Clinical Outcomes of Tenofovir Versus Zidovudine-based Regimens Among People Living with HIV / AIDS : a Two Years Retrospective. Open AIDS J 11: 1-11. https://bit.ly/2Y1JZQ4
- Awoke T, Worku A, Kebede Y, Kasim A, Birlie B, et al. (2016) Modeling outcomes of first-line antiretroviral therapy and rate of CD4 counts change among a cohort of HIV/AIDS patients in Ethiopia: A retrospective cohort study. PLoS One 11: 1-18. https://bit.ly/2YLc2QR
- Mbuagbaw LCE, Irlam JH, Spaulding A, Rutherford GW, Siegfried N (2010) Efavirenz or nevirapine in three-drug combination therapy with two nucleoside-reverse transcriptase inhibitors for initial treatment of HIV infection in antiretroviral-naïve individuals. Cochrane Database Syst Rev 12: CD004246. https://bit.ly/2Lgp1qV
- Bradley H, Mattson CL, Beer L, Huang P, Shouse RL (2009) infection 30: 2117–2124.
- Scarsi, Darin K, Rawizza K, Meloni H, Eisen S, et al. (2009) TDF-3TC-NVP is Inferior to AZT-3TC-NVP in a Large ART Program in Nigeria. Aids. 2010.
- Eyassu MA, Mothiba TM, Mbambo-Kekana NP (2016) Adherence to antiretroviral therapy among HIV and AIDS patients at the Kwa-Thema clinic in Gauteng Province, South Africa. African J Prim Heal Care Fam Med 8: 7. https://bit.ly/2LfK0dg
- Cain L (2012) The effect of efavirenz versus nevirapine-containing regimens on immunologic, virologic and clinical outcomes in a prospective observational study. AIDS 26: 1691-1705. https://bit.ly/2XVQdAX
- Nachega JB, Hislop M, Dowdy DW, Gallant JE, Chaisson RE, et al. (2008) Efavirenz versus nevirapine-based initial treatment of HIV infection: clinical and virological outcomes in Southern African adults. AIDS 22: 2117–2125. https://bit.ly/2YMdpis
- Ohene SA, Addo NA, Zigah F, Newman M, Lartey M, et al. (2013) Evaluation of antiretroviral therapy (ART) provision in an early cohort of patients initiating ART in Ghana. Pan Afr Med J 16: 117. https://bit.ly/2Y1kHSd
- Weiskopf D, Weinberger B, Grubeck-Loebenstein B (2009) The aging of the immune system. Transpl Int 22: 1041-1050. https://bit.ly/2XG23Lx
- Paintsil E (2011) Monitoring antiretroviral therapy in HIV-infected children in resource-limited countries: A tale of two epidemics. Vol. 2011, AIDS Research and Treatment. https://bit.ly/2XJ5HEu
- Langford SE, Ananworanich J, Cooper DA (2007) Predictors of disease progression in HIV infection: A review. AIDS Res Ther 4: 1–14. https://bit.ly/2LMvTvB
- Castetbon K, Anglaret X, Touré S, Chêne G, Ouassa T, et al. (2001) Prognostic value of cross-sectional anthropometric indices on short-term risk of mortality in human immunodeficiency virus-infected adults in Abidjan, Côte d’Ivoire. Am J Epidemiol 154: 75-84. https://bit.ly/2JA9r69
- Maas JJ, Dukers N, Krol A, Van Ameijden EJC, Van Leeuwen R, et al. (1998) Body mass index course in asymptomatic HIV-infected homosexual men and the predictive value of a decrease of body mass index for progression to AIDS. J Acquir Immune Defic Syndr Hum Retrovirology 19: 254–259. https://bit.ly/2YMqecz
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
