Archives of Clinical Gastroenterology
Prevalence of gastric cancer precursor lesions in patients of a secondary care center in a state in south of Brazil
Dominique Valentina Terebinto1*, Celso Nilo Didoné Filho1, Guilherme Ribas Taques2 and Michelle Gusmão de Assis3
2MD, Pathologist, Centro de Apoio em Patologia de Guarapuava (CAPG), Guarapuava, PR, Brazil
3MD, Pathologist, Centro de Anatomia e Patologia HISTOCENTER, Guarapuava, PR, Brazil
Cite this as
Terebinto DV, Didoné Filho CN, Taques GR, De Assis MG (2022) Prevalence of gastric cancer precursor lesions in patients of a secondary care center in a state in south of Brazil. Arch Clin Gastroenterol 8(1): 003-007. DOI: 10.17352/2455-2283.000105Copyright License
© 2022 Terebinto DV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and r eproduction in any medium, provided the original author and source are credited.Background: Atrophy of the gastric mucosa and intestinal metaplasia is considered malignant precursor lesions of gastric cancer, which is considered the fifth most common neoplasm in the world and the third cause of death from cancer. The main risk factor is the infection by Helicobacter pylori (H. pylori), which increases up to six times the risk of gastric cancer, through gastritis, atrophy, and hypochlorhydria, consequences of the infection. Other risk factors are also worth noting, like smoking and a family history of gastric cancer.
Objective: To investigate the prevalence of malignant precursor lesions and their associated factors in patients who underwent upper gastrointestinal endoscopy.
Methods: A descriptive, observational study was performed based on an analysis of endoscopic gastric biopsies performed in two affiliated private laboratories to the Unified Health System (Sistema Único de Saúde [SUS]) in a city in Paraná state. Patients were assessed for age, sex, active or recent smoking, family history of gastric cancer, and previous treatment for H. pylori. The samples were evaluated for the presence of glandular atrophy, intestinal metaplasia, dysplasia, and H. pylori infection.
Results: A total of 1,549 medical records and patient reports were evaluated and 945 were eligible, the average age was 52.2 (±14.3) years old and most patients (73.3%) were female. The prevalence of H. pylori infection was 47.5% (n= 449) and the highest percentage was between 30-39 years (58.7%). Among H. pylori-positive (+) patients who had developed intestinal metaplasia, there is more risk of having incomplete than complete metaplasia (OR: 4.34; 1.1–17.1; 95%CI). Patients who smoke are more increase the risk to developed glandular atrophy (OR: 1.91; 1.09-3.33; 95%CI) and intestinal metaplasia (OR: 1.93; 0.72-5.11; 95%CI).
Conclusion: The study reinforces risk factors such as smoking and H. pylori infection as precursors for developing pre-neoplastic lesions in a population in southern Brazil, highlighting the importance of smoking cessation and prevention of H. pylori infection and the treatment of infected patients.
Introduction
Adenocarcinomas are the predominant type of gastric cancer, corresponding to 95% of these neoplasms. This cancer is considered the fifth most common type of cancer in the world and the 3rd cause of death from cancer. In general, patients seek medical care when they have more evident symptoms, which indicate more advanced stages of the disease [1].
Among the main risk factors for the development of gastric cancer are smoking, Helicobacter pylori infection, and type of diet. Infection with Helicobacter pylori, a gram-negative bacterium, increases the risk of gastric cancer by up to six times [2-4]. The inflammation caused by the bacteria in the gastric mucosa gives rise to the process of carcinogenesis, being the first stage of chronic non-atrophic gastritis that progress with loss of the gastric glands, followed at intestinal metaplasia, occurring when there is a replacement of the glandular, superficial, and foveolar epithelium in the oxyntic or antral mucosa by the intestinal epithelium, and finally dysplasia that transforms into a gastric adenocarcinoma [1,5,6].
This process is made possible by the bacteria’s ability to survive under the acidic conditions of the stomach, creating resistance through synthesized enzymes, such as phospholipases and proteases that degrade mucus, who protects the gastric epithelium, as well as neutralizes the pH of the stomach that surrounds the bacteria. Furthermore, in response, inflammatory cells released from the host generate Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS), which react with DNA producing direct or indirect damage that contributes to mucosal damage [5-8].
The prognosis of gastric cancer is generally poor; this is probably due to its late diagnosis. Due to this factor, added to the knowledge of the development of the disease, it is pertinent to investigate the risk factors for premalignant lesions so that the diagnosis can be established early, with chances of increasing the life expectancy of patients [1,3]
From this review, the purpose of this paper was to evaluate the prevalence of malignant precursor lesions and their associated factors in patients who underwent an upper digestive endoscopy exam and who had gastric biopsy analyzed at two reference laboratories in Guarapuava City, Paraná State.
Methods
This descriptive, observational, individualized cross-sectional study was performed based on results from upper digestive endoscopies with biopsy collected between January 2019 and January 2021, in two pathology laboratories in the city of Guarapuava, which are also integrated into the Unified Health System. The clinical data of these patients were evaluated regarding sex, age, smoking status, and the presence of positive family history in a first-degree relative for gastric cancer.
The smoking status was considered positive when there was active smoking during the exam or at least one year previous to the exam.
Patients over 18 years of age who sought the secondary care service and who were submitted to upper endoscopy with samples of the gastric antrum and/or body and for whom H. pylori were investigated by specific staining were included in the study. For patients with more than one endoscopic exam with biopsies, only the samples corresponding to the first exam in the study period were considered. Patients under 18 years of age, those undergoing control endoscopy after treatment for H. pylori, who presented a different pattern in the sample, previous gastrectomy, also with biopsy compatible with gastric neoplasia, and those whose medical records were incomplete were excluded. As for the biopsy samples, the antrum and body samples were included simultaneously and separately.
The slides were stained by the GIEMSA method which shows satisfactory sensitivity, specificity, positive predictive value, and accuracy for bacillus identification [9].
Samples were evaluated for the presence of H. pylori in the antrum and/or gastric body (present or absent) and according to two morphologic variants: intestinal metaplasia and glandular atrophy, with these variables classified as absent or present, and when present ranked as mild, moderate and severe for glandular atrophy and complete or incomplete for intestinal metaplasia.
The Campo Real Ethics Committee approved this study and the report CAAE number is 38557620.0.0000.8947.
Statistical analysis
The Excell Microsoft Office Professional Plus 2013 program was used for data input and data analyses were performed with IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp. For statistical analysis, nominal data will be presented in frequencies with percentages and numerical data in mean with standard deviation. The differences in the distribution of variables were evaluated by the chi-square test and those with a P-value < 0.05 were considered statistically significant.
Results
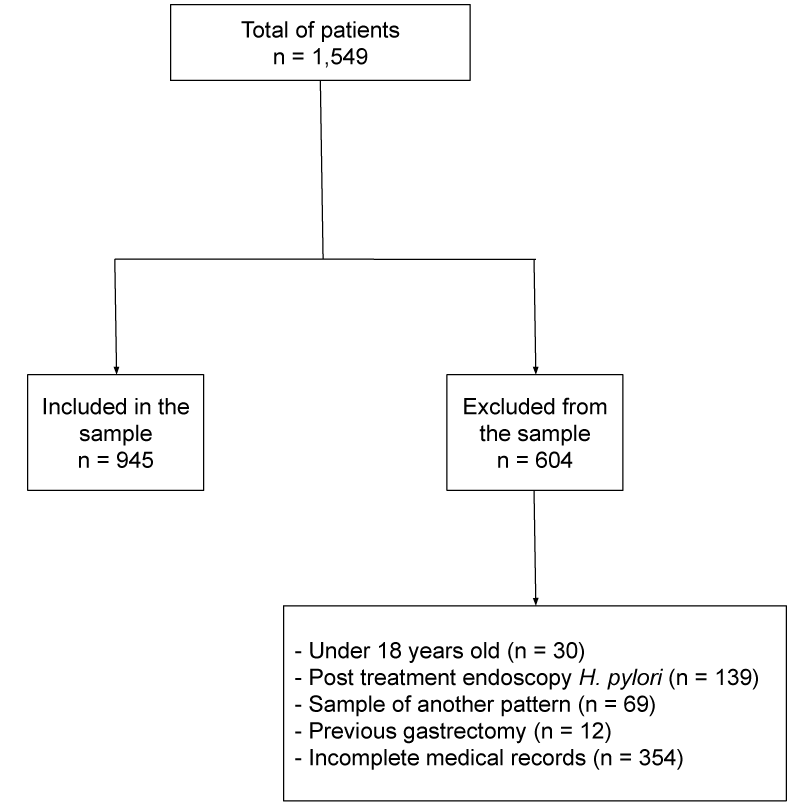
A total of 1,549 medical records and patient reports were analyzed and 945 met the inclusion criteria. Regarding the patients excluded from the sample, 30 were minors, 139 had post-treatment endoscopy for H. pylori, 69 the sample was of another pattern, 12 had already undergone the previous gastrectomy and 354 the medical record was incomplete (Figure 1). The average age of those included was 52.2 (±14.3) years old; the majority of them were women (n= 695; 73.5%). As shown in Table 1, the prevalence of H. pylori infection in this population was 47.5% (n= 449). The highest percentage of infection by H. pylori was between 30-39 years (58.7%), while the smallest percentage was detected among patients higher than 60 years (39.2%), a decrease was detected between 40-49 years of age followed by an increase between the patients with 50-59 years (p<0.01). The frequency of infection was higher among women (49.8%), however, there was no statistical significance (p= 0.47).
Of all the samples analyzed, 105 (11.2%) patients had some degree of intestinal metaplasia, 93 (9.8%) of them with complete metaplasia, and 12 (1.4%) with incomplete. Regarding glandular atrophy, 73 (7.8%) patients had some degree, with 45 (4.8%) mild atrophy, 25 (2.6%) moderate, and 3 (0.4%) severe. Of these patients, 24 (2.5%) had both metaplasia and atrophy in their sample. Regarding the risk factors surveyed during the study, 37 (3.9%) patients had a positive family history of gastric cancer and 100 (10.6%) patients were smokers.
When H. pylori were evaluated as a risk factor for the development of intestinal metaplasia, no statistical significance was found in the sample, with the odds ratio for the occurrence of metaplasia associated with H. pylori infection was 0.88 (0.58-1.32; 95%CI) not allowing association as a risk factor (Table 2). Patients with H. pylori infection who had developed intestinal metaplasia have a 3.4-fold increased risk of having incomplete than complete metaplasia (OR: 4.34; 1.1–17.1; 95%CI) (Table 3). When evaluating H. pylori as a risk factor for patients with glandular atrophy, there was no statistical significance in the sample, the odds ratio was 1.42, with a variance between 0.97 and 2.06 (Table 4).
Regarding smoking, when evaluated as a risk factor for the development of intestinal metaplasia, smoking patients have a 91% increased risk of developing metaplasia (OR: 1.91; 1.09–3.33; 95%CI) (Table 5). Similarly, when evaluated in relation to glandular atrophy, smoking patients have a 1.39-fold increase in the chance of developing atrophy (OR: 2.39; 1.3–4.4; 95%CI) (Table 6).
The risk of patients with a positive family history for gastric cancer to develop intestinal metaplasia, did not obtain statistical significance to make this statement (OR: 1.92; 0.92 – 4.5; 95%CI) (Table 7). Similarly, when compared to the risk of developing glandular atrophy, there was also no statistical significance (OR: 1.93; 0.72 – 5.11; 95%CI) (Table 8).
In addition, the coexistence of intestinal metaplasia, glandular atrophy, and positive and negative H pylori was also evaluated (Figure 2). It was observed that among patients with H. pylori (+), 6 (0.6%) had at least one type of precursor lesion, 398 (42.1%) had no metaplasia or atrophy and 24 (2.5%) had an H. pylori infection, atrophy, and metaplasia simultaneously. Among patients with H. pylori (-), 17 (1.8%) had a precursor lesion, 43 (4.5%) had both precursor lesions present, and the majority (n=436; 42.1%) did not show metaplasia or atrophy.
Discussion
In the present study, our objective was to demonstrate the epidemiological profile of the population in question and the risk factors that contribute to the development of pre-neoplastic lesions.
H. pylori is considered an important risk factor for the development of intestinal atrophy and metaplasia [10-12]. In developing countries, infection by H. pylori is very common, and about 50% of the world population is infected by this bacterium, which has a prevalence in Brazil of up to 71% [13,14]. In the current study, the prevalence of infection by H. pylori was 47.5%, being higher than in a previous study by Rodrigues et al. which was 31.7% in a different region of Brazil. This difference is probably due to the profile of the population studied (private x public health system).
When analyzing the epidemiological profile of the studied population, we observed a similar profile regarding gender and age to other studies such as Rodrigues et al., in addition to conferring a higher percentage of H. pylori infection between 30-39 years. In our study, the lowest percentage of H. pylori was in patients older than 60 years, unlike the study mentioned, in which the lowest percentage was in patients younger than 30 years. This difference can be explained by the sample size of the studies.
Regarding gastric atrophy and intestinal metaplasia, studies show that they are precursor lesions of gastric cancer [3,9,11]. A systematic review and meta-analysis analyzed the prevalence of intestinal atrophy and metaplasia in the general population, including 107 original articles comparing countries with high versus low to the moderate incidence of gastric cancer [12]. In the cited review, there was a higher prevalence of atrophy in the general population than in intestinal metaplasia. When compared to countries with a high incidence of this neoplasm, the prevalence of atrophy was 41.7%, while in countries with a low to moderate incidence it was 22.8%. Regarding metaplasia, 21.7% presented in countries with low to moderate incidence of gastric cancer and 28.1% in countries with high incidence, with no significant difference between these countries.
In the present study, the prevalence of metaplasia was 11.2% and of atrophy was 7.8%, being lower than expected for countries with a low incidence of gastric cancer. However, these numbers follow the same direction as some studies already carried out in Brazil and were in agreement with a retrospective study carried out in the southeast region of Brazil, in which the prevalence of intestinal metaplasia and atrophy was, respectively, 14.7% and 10.3% [6].
Some studies show that the risk of gastric cancer may be greater in patients with incomplete-type metaplasia than in those with the complete-type, in addition to increasing the risk in patients with a family history of gastric cancer in a first-degree relative [16-19]. A meta-analysis of seven studies demonstrated that having incomplete versus complete metaplasia is associated with a three-fold increased risk for developing gastric cancer (over 3 to 13 years follow-up; Relative Risk [RR] 3.3, 95%CI, 1.9 -5.6) [20]. When evaluating the prevalence of intestinal atrophy and metaplasia in relation to infection by H. pylori, there was no statistical significance in our study, but patients who had H. pylori infection and metaplasia had a higher risk of developing incomplete than complete metaplasia.
When evaluating the prevalence of pre-neoplastic lesions in relation to smoking, we saw that patients who smoke are at increased risk for the development of atrophy (OR: 2.39; 1.3–4.4; 95%CI) and metaplasia (OR: 1.91; 1.09–3.33; 95%CI), thus increasing the chance of developing gastric cancer, since smoking is an important risk factor for this condition [15].
A family history of gastric cancer is also considered a risk factor for cancer [15,17], but in the present study, it was not possible to associate this factor with the development of pre-neoplastic lesions.
Smoking cessation and H. pylori eradication are measures that decrease the risk of gastric cancer, although eradication of the infection does not completely regress metaplasia and atrophy, it can delay the development of these lesions and progression to gastric cancer [10,21,22].
We considered the study’s limiting factors, the low replicability of the sample, as it was limited to only one city in the southern region of Brazil, another limitation is the sample only from the gastric body or only from the antrum.
Conclusion
The present study contributes to a better understanding of the risk factors associated with the development of pre-neoplastic lesions in a population in southern Brazil, which include smoking and H. pylori infection. In the study of this population, we concluded that patients aged between 30-39 years are more infected with H pylori and that smokers predispose to the development of intestinal atrophy and metaplasia, thus being a population with a greater chance of developing gastric cancer. We also conclude that there is an increased risk of developing incomplete-type metaplasia in this population, further increasing the chance of gastric cancer. Thus, we reinforced measures such as smoking cessation and the prevention of H. pylori infection, as well as the treatment of infected patients.
Authors’ contribution
Terebinto DV: Initial literature review, data collection, statistical analysis, and writing of the text. Filho CND: data collection, statistical analysis, and text review. Takes GR: supply of samples and analysis of biopsies. Assis MG: supply of samples and analysis of biopsies.
- Jeong S, Choi E, Petersen CP, Roland JT, Federico A, et al. (2017) Distinct metaplastic and inflammatory phenotypes in autoimmune and adenocarcinoma‐associated chronic atrophic gastritis. United European Gastroenterol J 5: 37-44. Link: https://bit.ly/3HAgxUR
- Lee YC, Chiang TH, Chou CK, Tu YK, Liao WC, et al. (2016) Association between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology 150: 1113-1124.e5. Link: https://bit.ly/3ryCxKd
- Park YH, Kim N (2015) Review of Atrophic Gastritis and Intestinal Metaplasia as a Premalignant Lesion of Gastric Cancer. J Cancer Prev 20: 25–40. Link: https://bit.ly/3J5tZR0
- Shao L, Li P, Ye J, Chen J, Han Y, et al. (2018) Risk of gastric cancer among patients with gastric intestinal metaplasia. Int J Cancer 143: 1671–1677. Link: https://bit.ly/34GmpNI
- Choi IJ, Kim CG, Lee JY, Kim YI, Kook MC, et al. (2020) Family History of Gastric Cancer and Helicobacter pylori Treatment. N Engl J Med 382: 427–436. Link: https://bit.ly/3sliMFn
- Rodrigues MF, Guerra MR, Alvarenga AVR de, Souza DZ de O, Costa RAV S, et al. (2019) Helicobacter pylori infection and gastric cancer precursor lesions: prevalence and associated factors in a reference laboratory in southeastern Brazil. Arq Gastroenterol 56: 419–424. Link: https://bit.ly/3sliMFn
- Jain U, Saxena K, Chauhan N (2021) Helicobacter pylori induced reactive oxygen Species: A new and developing platform for detection. Helicobacter 26: e12796. Link: https://bit.ly/3oznD4K
- Chatre L, Fernandes J, Michel V, Fiette L, Avé P, Arena G, et al. (2017) Helicobacter pylori targets mitochondrial import and components of mitochondrial DNA replication machinery through an alternative VacA-dependent and a VacA-independent mechanisms. Sci Rep 7: 15901. Link: https://bit.ly/3uy8YKF .
- Pandya HB, Patel JS, Agravat HH, Patel SB, Thakkar MC (2013) Identification of Helicobacter pylori by different conventional staining techniques and its comparison with polymerase chain reaction. Saudi Med J 34: 942-948. Link: Link: https://bit.ly/3J80xKg
- Choi AY, Strate LL, Fix MC, Schmidt RA, Ende AR, Yeh MM, et al. (2018) Association of gastric intestinal metaplasia and East Asian ethnicity with the risk of gastric adenocarcinoma in a U.S. population. Gastrointest Endosc 87: 1023–1028. Link: https://bit.ly/3B3vSLa
- Mera RM, Bravo LE, Camargo MC, Bravo JC, Delgado AG, et al. (2018) Dynamics of Helicobacter pylori infection as a determinant of progression of gastric precancerous lesions: 16-year follow-up of an eradication trial. Gut 67: 1239-1246. Link: https://bit.ly/3B9qM0e.
- Spence AD, Cardwell CR, McMenamin ÚC, Hicks BM, Johnston BT, et al. (2017) Adenocarcinoma risk in gastric atrophy and intestinal metaplasia: a systematic review. BMC Gastroenterol 17: 157. Link: https://bit.ly/3LaHkJE
- Marques-Silva L, Areia M, Elvas L, Dinis-Ribeiro M (2014) Prevalence of gastric precancerous conditions: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol 26: 378–387. Link: https://bit.ly/3os5W75
- Goderska K, Agudo Pena S, Alarcon T (2018) Helicobacter pylori treatment: antibiotics or probiotics. Appl Microbiol Biotechnol 102: 1-7. Link: https://bit.ly/3uw6w7F
- Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, et al. (2017) Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 153: 420–429. Link: https://bit.ly/3HD3rWX
- Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F (2020) Gastric cancer. Lancet 396: 635-648. Link: https://bit.ly/3oyfrl1
- González CA, Sanz-Anquela JM, Gisbert JP, Correa P (2013) Utility of subtyping intestinal metaplasia as marker of gastric cancer risk. A review of the evidence. Int J Cancer 133: 1023-1032. Link: https://bit.ly/3JaU4y3
- den Hollander WJ, Holster IL, den Hoed CM, Capelle LG, Tang TJ, et al. (2019) Surveillance of premalignant gastric lesions: a multicentre prospective cohort study from low incidence regions. Gut 68: 585-593. Link: https://bit.ly/3GEGC3L
- González CA, Pardo ML, Liso JMR, Alonso P, Bonet C, et al. (2010) Gastric cancer occurrence in preneoplastic lesions: a long-term follow-up in a high-risk area in Spain. Int J Cancer 127: 2654–2660. Link: https://bit.ly/3rAQpny
- González CA, Sanz-Anquela JM, Companioni O, Bonet C, Berdasco M, et al. (2016) Incomplete type of intestinal metaplasia has the highest risk to progress to gastric cancer: results of the Spanish follow-up multicenter study. J Gastroenterol Hepatol 31: 953-958. Link: https://bit.ly/338oy4C
- Gawron AJ, Shah SC, Altayar O, Davitkov P, Morgan D, et al. (2020) AGA Technical Review on Gastric Intestinal Metaplasia-Natural History and Clinical Outcomes. Gastroenterology 158: 705-731.e5. Link: https://bit.ly/335K367
- Wang J, Xu L, Shi R, Huang X, Li SWH, et al. (2011) Gastric atrophy and intestinal metaplasia before and after Helicobacter pylori eradication: a meta-analysis. Digestion 83: 253-260. Link: https://bit.ly/3HAgFnj
- Zhou L, Lin S, Ding S, Huang X, Jin Z, Cui R, et al. (2014) Relationship of Helicobacter pylori eradication with gastric cancer and gastric mucosal histological changes: a 10-year follow-up study. Chin Med J 127: 1454-1458. Link: https://bit.ly/3GzADNG
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
