Archives of Clinical Gastroenterology
Clinical effects of Lactobacillus strains as probiotics in the treatment of irritable bowel syndrome. Results from the LAPIBSS trial: Future objectives
Jean-Michel Maixent1,2*, Olivier Pons2, Souad R Sennoune3 and Stéphane Sadrin4*
2IAPS Laboratory, University of Toulon, Toulon, France
3Department of Cellular Physiology and Molecular Biophysics, Texas Tech University Health Sciences Center, Lubbock, USA
4Laboratoire Denel-Codifra, Le Chesnay, France
Cite this as
Maixent JM, Pons O, Sennoune SR, Sadrin S (2020) Clinical effects of Lactobacillus strains as probiotics in the treatment of irritable bowel syndrome. Results from the LAPIBSS trial: Future objectives. Arch Clin Gastroenterol 6(2): 031-035. DOI: 10.17352/2455-2283.000075The objective of this communication is to present and analyze the recent results from the LAPIBSS study in order to improve future clinical trials on the effects of Lactobacillus strains in the treatment of irritable bowel syndrome (IBS). Using a tightly-controlled clinical trial protocol with the highest Jadad score of 5/5, the current trial aimed to demonstrate the efficacy of a 2-strain mixture of Lactobacillus acidophilus to improve IBS symptoms. Eighty patients diagnosed for IBS according to Rome III criteria were recruited to a multicentric, double-blinded, in parallel groups, placebo-controlled, randomized trial. Patients were provided with a daily dose of two capsules containing either two probiotic strains (5×109cfu/capsule) or placebo for 8 weeks. The primary endpoint was abdominal pain score assessed with a 100-mm visual analogue scale (VAS). Secondary endpoints included scores of bloating, flatus and rumbling assessed with a 100-mm VAS, a composite score that consisted of the sum of the 4 VAS scores, and the stool frequency and consistency assessed with the Bristol Stool Form Scale. Our study has failed to demonstrate a significant improvement of the primary endpoint of abdominal pain. Significant differences between groups were observed for flatus score at week 4 (P= 0.04) and week 8 (P= 0.03) and for composite score at week 8 (P= 0.04). The consumption of the 2-strain mixture of L. acidophilus over 8 weeks is safe, significantly decreases flatus and IBS composite scores. The significant effect on flatus could result from the species-specific homofermentative properties of L. acidophilus strains. The negative results on abdominal pain and the gained experience are discussed for the future clinical trials on this topic.
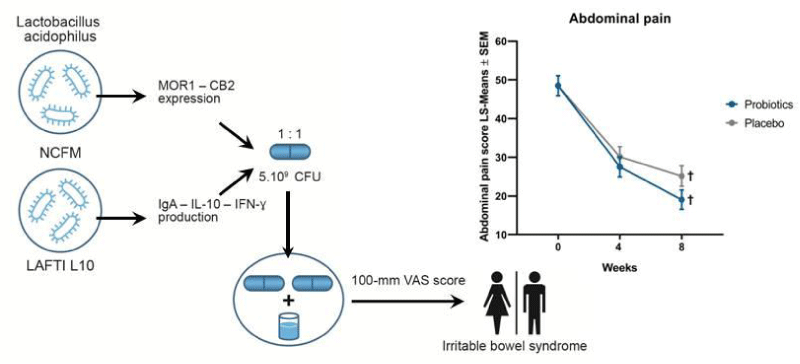
Irritable Bowel Syndrome (IBS) is a functional gastrointestinal disorder and consists of chronic and recurrent abdominal pain associated with defecation or a change in bowel habits including diarrhea and/or constipation [1,2]. A recent systematic review performed by Chey et al. (2015) concluded that diagnosis of IBS is based on the identification of characteristic symptoms and the exclusion of other organic diseases [3]. As this review concerns medical education, its associated quiz for medical education is a useful prerequisite for physicians performing clinical trials in IBS [3]. Due to the lack of a well-established therapeutic approach, IBS patients seek alternative strategies such as probiotics [4]. Several food-associated Lactobacillus species have an excellent safety profile and have a “generally-regarded-as-safe” (GRAS) status, such as Lactobacillus acidophilus (L. acidophilus) [5]. While L. acidophilus is one of the most commonly dietary used bacterial species, only 2 randomized clinical trials (RCT) performed with L. acidophilus strains and conducted with a high-quality method have been reported in the management of IBS symptoms [5-8]. The first one was a pilot RCT including 40 patients and showed that L. acidophilus-SDC 2012, 2013 significantly reduced abdominal pain compared with placebo after 4 weeks of treatment [7]. Recently, a 12-week RCT performed with L. acidophilus NCFM showed significant improvement of abdominal pain in the subgroup of patients suffering from moderate to severe pain [8]. Using a tightly-controlled protocol firstly published as Lactobacillus acidophilus versus placebo in the symptomatic treatment of irritable bowel syndrome (LAPIBSS), we conducted a RCT investigating for a 2-month period the safety and efficacy of a 2-strain mixture of L. acidophilus to manage IBS symptoms which has provided the following positive results and conclusion [9,10]. 1/ at the end of the trial, probiotics reduced significantly flatus and composite scores, 2/ this RCT based on strain-specific properties confirmed the safety of probiotics and showed their limited efficacy to improve IBS symptoms, 3/ the beneficial effect on flatus severity could result from the species-specific homofermentive properties of L. acidophilus strains. The LAPIBSS protocol suggested an additional benefice of a combined treatment with two strains of the same species without known antagonist effects [9]. The rationale for combining L. acidophilus NCFM with a second available L. acidophilus strain, i.e. L. acidophilus subsp. helveticus LAFTI L10, was based on preclinical and clinical evidence for their strain-specific properties targeting the intestinal system [11,12]. However, taking into account the multifactorial pathophysiology of IBS, the strong placebo effect observed in this trial could warrant further study with probiotics targeting the gut-brain axis [3,4].
The LAPIBSS protocol refers to a multicentric (10 office-based general practitioner located in France and one gastroenterologist of the Rangueil University Hospital of Toulouse, France), double-blind, placebo-controlled, two-armed, parallel design, individually randomized trial, comparing probiotics with placebo in patients with IBS aged between 30 and 60 years old [9]. The current trial was performed for a maximum of 9 weeks with 4 visits planned (at points corresponding to screening, baseline, 2 control visits after 4 and 8 weeks of treatment) [9]. Patients were diagnosed for IBS according to Rome III criteria with in addition a negative coprological and inflammatory balance (negative CRP blood test) for over 6 months [9]. Other selection criteria were detailed in the LAPIBSS protocol [9].
The study product was provided in the form of vegetable capsule containing a blend of two viable lyophilized L. acidophilus strains: L. acidophilus NCFM (FDA GRAS Notice 000357, strain number ATCC SD5221, Danisco Inc. Madison, Wisconsin, United States) and L. acidophilus subsp. helveticus LAFTI L10 (strain number CBS 116.411, Lallemand Health Solutions, Blagnac, France). This mixture of two probiotic strains provides for each 2.5×109 colony-forming unit (cfu) for a total of 5×109 cfu per capsule. The trial dose was 2 capsules/day taken orally; one in the morning and the other one in the evening with a full glass of water half an hour before eating.
IBS symptoms of abdominal pain, bloating, flatus and rumbling were recorded by the clinical investigator for each patient at the baseline visit and at both visits (weeks 4 and 8). Each IBS symptom score was assessed with a 100-mm visual analogue scale (VAS; 0: none; 100: very severe) [13]. The composite score was the sum of 4 VAS scores (abdominal pain, bloating, flatus and rumbling) calculated for each patient.
For a relevant comparison with the LAPIBSS protocol, the Table 1 shows the characteristics of the high-quality RCT investigating the benefits of Lactobacillus strains on patients with IBS [8]. Only the multicentric clinical trials have been selected and reported. A similar table including the monocentric trials or was presented in our previously published study protocol [9].
The objective of this communication is to present and analyse the recent results from the LAPIBSS study and to improve the design of future RCT investigating the clinical effects of Lactobacillus strains on IBS symptoms. Using a tightly-controlled study protocol with the highest Jadad score of 5/5 (Table 1), the current trial aimed to demonstrate the efficacy of a 2-strain mixture of L. acidophilus to improve IBS symptoms but remains negative on the abdominal pain score used as primary endpoint (Figure 1). Significant differences between groups were observed for the following secondary endpoints: flatus score at week 4 (P=0.04) and week 8 (P=0.03) and composite score at week 8. (P=0.04) [10]. The consumption of the 2-strain mixture of L. acidophilus over 8 weeks is safe confirming the GRAS status of Lactobacilli [5]. A mode of action at the molecular level for the significant effect observed on flatus score could result from the species-specific homofermentative properties of L. acidophilus strains able to produce lactic acid without gas production [5]. One possible improvement of the next clinical study should be the use of an appropriate dosage of the Lactobacillus strains (an insufficient dosage is a limiting factor) and/or the duration of the trial (at least 12 weeks or more, up to 6 months).
The dose-response relationship with the probiotic mixture or with each stain used alone should complete this first clinical study especially because one of the two Lactobacillus strains, i.e. L. acidophilus NCFM, was chosen due to its strain-specific mechanism of action on μ1-opioid receptor (MOR1) and cannabinoid receptor 2 (CB2) expression in intestinal epithelial cells that could be responsible for the decrease of the abdominal pain severity perceived by the patients with IBS [11]. The investigation of the dose-response relationship needs further RCT as outlined by EFSA guidelines [14]. Stool samples could be collected to ascertain compliance with a potential change in its microbial composition. This would increase the level of evidence to demonstrate the clinical effects (treatment benefits) of probiotics for IBS [14].
Another important observation and objective will be to decrease the placebo response rate as reported by the meta-analysis performed by Ford et al. (2010) [15]. The placebo effect in IBS is a constant preoccupation since the meta-analysis on the placebo response rate in IBS trials done by Patel et al. (2005) analysing the magnitude of responses in placebo arms within 11 variables [16]. Two variables that could independently decrease the placebo response were outlined consisting in using the Rome criteria and in increasing the number of visits [16].
We have taken these factors into consideration by using Rome criteria for patient enrolment and in the establishment of the number of visits during the preparation of the study protocol [9]. In our study, the available Rome III criteria were used without success since the magnitude of placebo responses remains high supporting the enrolment of IBS patients according to the recently defined Rome IV diagnosis criteria [1,10]. With regard to increasing the number of visits, it was possible in theory to extend from the minimum of 1 visit (2 weeks) to 12 visits (12 weeks) but we used 3 visits (8 weeks) in our study [9,16]. Indeed, it seems in our view difficult to extend to 1 visit/week for a health food supplement such as probiotics to implement this schedule both for office-based general practitioners and patients.
Abdominal pain for example is assessed by a VAS score which is always difficult to follow over a long-term period. Furthermore, the use of VAS remains a subjective method. Hence, an objective assessment method (by novel biomarkers) is required to measure abdominal pain severity and the others IBS symptoms except for stool consistency assessed with the validated Bristol Stool Form Scale which is fulfilled by the patient itself [17].
The strong placebo effect in IBS, as evidenced by the meta-analysis done by Patel et al. [16], should be reinvestigated with its consideration as a placebo analgesia (mediated by endogeneous opioid release), with new Rome IV criteria and taking account of the potential heterogeneity of global population of patients with IBS by the achievement of RCT selecting participants according to the IBS subtypes (diarrhoea, constipation or mixed)1. This may entail a confounding factor and contribute to the lack of significant differences for IBS symptoms, especially for bowel-related symptoms. A better understanding of the physiological effect of probiotics in human microbiota would provide a refined rationale for their use prior to future clinical trials for IBS. Representative patients of the real-life situations and multicentric trials are also required (Table 1) [9].
The clinical effects assessment and the rationale for the use of Lactobacillus strains and probiotics in general could also be based on the use of validated IBS Symptom Severity Score (IBS-SSS) and IBS-related quality of life questionnaires [18-22]. Other surveys including variables diet (with dietitian), allergens, food intolerance as previously suggested for physicians [3], are also of interest for future IBS clinical protocols.
In conclusion, the therapeutic interest of Lactobacillus strains used as health food supplements to improve IBS symptoms remains limited by the low number of high-quality RCT and could be improved by deeper research and knowledge on the IBS.
Conflicts of interest: SS is an employee of Laboratoire Denel-Codifra (Le Chesnay, France), which supplied probiotics and the placebo for the research. The remaining authors disclose no competing interests.
Aknowlegments: Dr Raul Martínez-Zaguilán (Texas Tech University Health Sciences Center, Lubbock, USA) for the proofing of the manuscript.
Author contributions: SS, OP,SRS and JMM designed the research study. JMM and SS wrote the manuscript.
Declaration of funding: This study was funded by OSEO Innovation - Bpifrance (Maisons-Alfort, France) and Laboratoire Denel-Codifra (Le Chesnay, France).
- Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, et al. (2016) Bowel Disorders. Gastroenterology. Link: https://bit.ly/3fY8C6G
- Palsson OS, Whitehead WE, van Tilburg MA, Chang L, Chey W, et al. (2016) Rome IV Diagnostic Questionnaires and Tables for Investigators and Clinicians. Gastroenterology. Link: https://bit.ly/2zEsxH4
- Chey WD, Kurlander J, Eswaran S (2015) Irritable bowel syndrome: a clinical review. JAMA 313: 949-958. Link: https://bit.ly/2yaMLru
- Simrén M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, et al. (2013) Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut 62: 159-176. Link: https://bit.ly/2WFyCMx
- Bull M, Plummer S, Marchesi J, Mahenthiralingam E (2013) The life history of Lactobacillus acidophilus as a probiotic: a tale of revisionary taxonomy, misidentification and commercial success. FEMS Microbiol Lett 349: 77-87. Link: https://bit.ly/2WYSl8N
- Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P (2018) Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther 48: 1044-1060. Link: https://bit.ly/3dPclBp
- Sinn DH, Song JH, Kim HJ, Lee JH, Son HJ, et al. (2008) Therapeutic effect of Lactobacillus acidophilus-SDC 2012, 2013 in patients with irritable bowel syndrome. Dig Dis Sci 53: 2714-2718. Link: https://bit.ly/2WDZgW2
- Lyra A, Hillilä M, Huttunen T, Männikkö S, Taalikka M, et al. (2016) Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J Gastroenterol 22: 10631-10642. Link: https://bit.ly/3bx4vLe
- Sadrin S, Sennoune SR, Gout B, Marque S, Moreau J, et al. (2017) Lactobacillus acidophilus versus placebo in the symptomatic treatment of irritable bowel syndrome: the LAPIBSS randomized trial. Cell Mol Biol (Noisy-le-grand) 63: 122-131. Link: https://bit.ly/3bzV91q
- Sadrin S, Sennoune S, Gout B, Marque S, Moreau J, et al. (2020) A 2-strain of Lactobacillus acidophilus p is effective in the treatment of irritable bowel syndrome: A placebo-controlled, randomized clinical trial. Dig Liver Dis 52: 534-540. Link: https://bit.ly/3bzV91q
- Rousseaux C, Thuru X, Gelot A,Barnich N, Neut C, et al. (2007) Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med 13: 35-37. Link: https://bit.ly/2yaVKsI
- Paturi G, Phillips M, Jones M, Kailasapathy K (2007) Immune enhancing effects of Lactobacillus acidophilus LAFTI L10 and Lactobacillus paracasei LAFTI L26 in mice. Int J Food Microbiol 115: 115-118. Link: https://bit.ly/3653TeF
- Design of Treatment Trials Committee, Irvine EJ, Whitehead WE, Chey WD, Matsueda K, et al. (2006) Design of treatment trials for functional gastrointestinal disorders. Gastroenterology 130: 1538-1551. Link:
https://bit.ly/3dLsKXq - EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) (2016) Guidance on the scientific requirements for health claims related to the immune system, the gastrointestinal tract and defence against pathogenic microorganisms. EFSA Journal 14: 4369. Link: https://bit.ly/2TbSLHM
- Ford AC, Moayyedi P (2010) Meta-analysis: factors affecting placebo response rate in the irritable bowel syndrome. Aliment Pharmacol Ther 32: 144-158. Link: https://bit.ly/2X1palp
- Patel SM, Stason WB, Legedza A, Ock SM, Kaptchuk TJ, et al. (2005) The placebo effect in irritable bowel syndrome trials: a meta-analysis. Neurogastroenterol Motil 17: 332-340. Link: https://bit.ly/368Jo0S
- Lewis SJ, Heaton KW (1997) Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 32: 920-924. Link: https://bit.ly/3fWcF31
- Francis CY, Morris J, Whorwell PJ (1997) The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 11: 395-402. Link: https://bit.ly/3dOLvte
- Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL (1998) Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci 43: 400-411. Link: https://bit.ly/2WA5cz6
- Niv E, Naftali T, Hallak R, Vaisman N (2005) The efficacy of Lactobacillus reuteri ATCC 55730 in the treatment of patients with irritable bowel syndrome – a double-blind, placebo-controlled, randomized study. Clin Nutr 24: 925-931. Link: https://bit.ly/3g47TRo
- Ducrotté P, Sawant P, Jayanthi V (2012) Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol 18: 4012-4018. Link: https://bit.ly/3bE7YI2
- Dapoigny M, Piche T, Ducrotté P, Lunaud B, Cardot JM, et al. (2012) Efficacy and safety profile of LCR35 complete freeze-dried culture in irritable bowel syndrome: a randomized, double-blind study. World J Gastroenterol 18: 2067-2075. Link: https://bit.ly/2WZXGg1
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
