Archives of Clinical Gastroenterology
Incomplete Currarino Syndrome: Case Report and a Brief Review of Literature
Muhammad Abdelhafez Mahmoud*and Ashraf Hamed Seddek
Cite this as
Mahmoud MA, Seddek AH (2020) Incomplete Currarino Syndrome: Case Report and a Brief Review of Literature. Arch Clin Gastroenterol 6(1): 013-016. DOI: 10.17352/2455-2283.000070Currarino Syndrome (CS) or triad is rare entity that was first reported by Currarino in 1981 as a pathology involving three anomalies; anorectal malformation, a sacral bony defect and a presacral mass. CS can be familial or sporadic, with an autosomal dominant inheritance mode. The English literature reported over 310 cases of Currarino triad with the cases diversity in age, clinical presentation, and management.
Herein, we present a case of neonate with incomplete CS (presacral mass [Sacrococcygeal Teratoma SCT] and low anorectal malformation with normal sacrum). He was diagnosed antenatally. He was full term, weighing 3250 gm and delivered by Caeserian section. He was operated on the second day of life. According to Altman’s classification, the case had type II SCT (mainly external with small intrapelvic component). The sacrococcygeal teratomatous mass was about 13×9×10 cm in diameter and weights about 950 gm. He underwent combined abdominal/posterior sacral approach. He underwent diverting colostomy via abdominal exploration & complete excision of the sacrococcygeal mass together with the coccyx through chevron posterior approach, and finally anorectoplasty was done all in the same setting. Colostomy was closed 4 months later and tumor marker turned normal and the child has grown well and led a normal life with accepted continence with no recurrence after 4.8 years of follow up.
Conclusion: We report a case of incomplete Currarino’s syndrome comprising imperforate anus and sacrococcygeal teratoma (with pre-sacral extension) but lacking the bony sacral anomaly component of the triad.
Introduction
The first case of CS triad was reported by Currarino, et al. in 1981 as a disorder involving three anomalies; anorectal malformation (usually anorectal stenosis or anteriorly displaced anus), bony sacral defect and a presacral mass. Currarino triad is an autosomal dominant inherited pathology resulting from abnormal separation of neuroectoderm from endoderm due to a mutation in the HLXB9 (renamed MNX1) homeobox gene present on chromosome 7q36 discovered by Lynch & colleagues at 1995, found in about all familial cases and up to one third of the sporadic cases as reported by Ross, et al. in 1998 [1]. CS-related lesions exist in about 60%-90% of the affected cases family members. Over 310 cases of CS have been reported in the literature. Most cases are familial, with estimated incidence about 1 in 100.000 population but the exact incidence is unknown because most cases information came from tertiary care referral centers as case reports [2].
High level of AFP is normal in the first 8-10 months of life while the average time needed for AFP to return back to normal after SCT excision is about 9 months. Early resection of SCT plus the coccyx is the operative procedure of choice for treating this tumor [3,4].
Case details
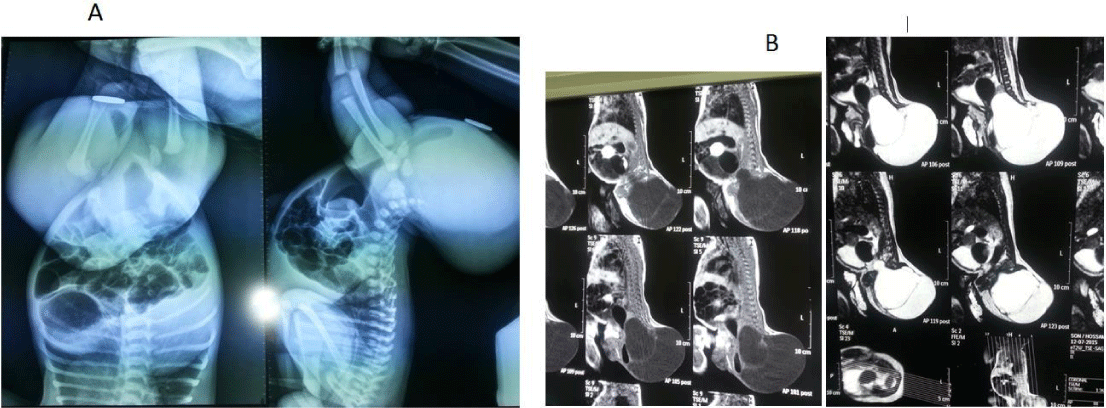
In this article, we present a neonate male baby with incomplete Currarino syndrome (presacral mass [sacrococcygeal teratoma] and anorectal malformation) as in Figure 1. The baby was investigated by basic lab investigations, invertogram that revealed that it was low ARM (Figure 2A), MRI abdomen/pelvis/mass which did not reveal tethered cord or any other cord anomalies (Figure 2B), & alpha-fetoprotein level. Gene test was not done with no family history of similar conditions meaning that it was a sporadic case.
Under general endotracheal anesthesia, sterilization done and the baby was prepped and draped. He was firstly positioned supine for abdominal exploration part of the operation then shifted to prone jack-knife position for posterior sacral part of the operation. The operative steps were as follows:
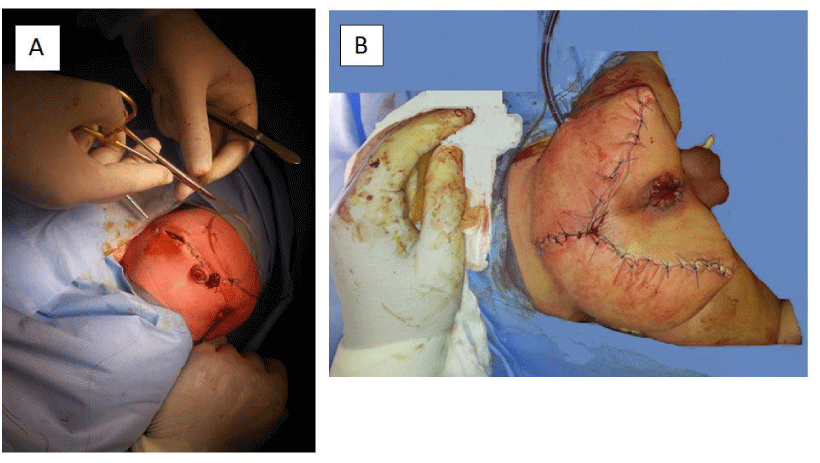
Abdominal exploration through right transverse supra-umbilical incision as per the surgeon’s preference was done for evaluation of the intestine and intrapelvic mass, vascular control of the SCT, secure umbilical vessel catheter placement for vascular access, and defunctioning colostomy (at the junction between descending and sigmoid colon) was done (Figure 3A). An umbilical artery catheter was inserted at the beginning of operation. Chevron (Inverted V shaped) incision was done for excision of the sacrococcygeal teratomatous mass.
- Deepening of the incision to reach the tumour capsule
- Raising the skin flaps to expose the tumour completely
- Dissection of the tumour from the inferior and medial aspects of the gluteus maximus muscle to visualize the sacrum and coccyx
- Transection of the coccyx at the sacrococcygeal joint
- Identification of the rectum
- Dissection of the tumour from the rectal wall
- Completion of ventral dissection and the mass was removed completely including the intrapelvic component from the same incision
- Anchoring of the levator muscles to the presacral fascia
- Approximating gluteal muscles
- Completion of the suture line (Figure 3B)
- Insertion of a drain in the operative bed.
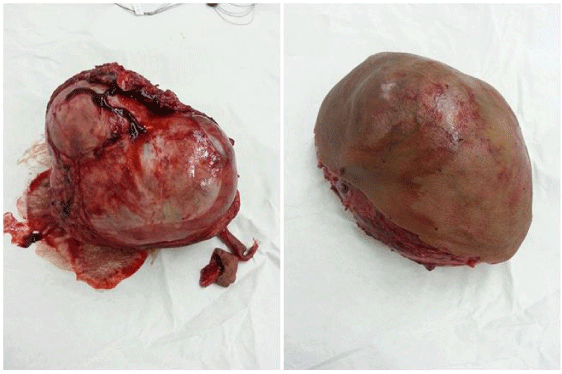
The mass was removed completely via the chevron incision. Anorectoplasty also was done. Rectal biopsy was taken and was found ganglionic. The excised specimen of sacrococcygeal teratomatous mass was about 13x9x10 cm in diameter (Figure 4) and weighs about 950 gm. Histopathology of the mass revealed mature (benign) teratoma with no need for post-operative chemotherapy. Alpha-fetoprotein was 10.000 ng/dl pre-operatively and dropped to 2.000 ng/dl one month post-operatively. The post-operative course was smooth and uneventful and the baby was discharged from the hospital after 2 days in stable good health status. Colostomy was closed 4 months later and tumor marker turned normal and the child has grown well. Anal sphincter/pelvic EMG and colonic transit time were done in follow up to assess bowel function and found normal with accepted continence and good voluntary bowel movement as also concluded from history and physical examination in OPD visits. Urinary control was also normal. Quarterly abdominoplevic US and annual MRI revealed no recurrence for 4.8 years of follow up.
Discussion
Currarino syndrome is a rare congenital complex syndrome that was first described by Kennedy in the mid-twenties of the last century [5] and further reported by and named after Guido Currarino, et al. [6], (a pediatric radiologist) in 1981, characterized by a sacral bone defect, which is diagnostic in case of a sickle-shaped sacrum (hemisacrum); a congenital hindgut anomaly; in one third of cases it is rectal stenosis; and a presacral tumour, which is an anterior meningocele in less than two thirds of patients. Half of cases are due to an autosomal dominant mutation in the HLXB9 (MNX1) gene (on chromosome 7q36). This gene is involved in development of the caudal end of embryo and its mutations cause the abnormal separation of the neuroectoderm and endoderm before the development of the notochord. Currarino syndrome is a unique form of caudal regression syndrome [7-10].
The phenotype clinical presentation is diverse as it can be incomplete with 1 or other anomaly absent in the triad making the diagnosis easily missed as some patients may only present with symptomless sacral anomaly or complain of mild constipation not fully evaluated [11,12].
Deletion mutations involving 7q chromosome cause the typical scimitar sacrum (hemisacrum), present in nearly all familial cases and in some sporadic cases [1]. With increasing Currarino syndrome patient number, multidisciplinary diagnostics and therapeutic guidelines are being proposed [13-15].
The external effects of the gene mutations causing the CS can vary from asymptomatic cases to patients presenting with the complete full-blown triad [16]. So, the number CS patients have been much underestimated. About 50%-60% of the reported cases have a positive family history of triad anomalies [17]. A teratoma is a neoplasm originating from primordial germ cells and is derived from all three embryonal germ layers. Actually, sacral hypoplasia could remain asymptomatic in many examples. Lynch and colleagues found that 16% of patients with CS manifest with infantile bowel obstruction. Patients with CS commonly present with other anomalies including urological disorders such as congenital single kidney, hydronephrosis, vesicoureteric reflux, voiding dysfunction, urinary incontinence, duplex ureters and tethered cord [11].
Anterior meningocele represents approximately 50% of presacral masses, teratoid tumor represents 40%, and the remaining includes neuroeneteric, anal duplication cyst, immature teratoma, hamartoma, nephroblastoma, primitive Neuroendocrine Tumor (pNET), epidermoid, dermoid cyst, or lipoma. It is scarcely accompanied by malignant teratoma [2,8].
The English literature on isolated Sacrococcygeal Teratoma (SCT) contains only two reports of adults with multiple synchronous teratomas. Recurrence after grossly complete excision of a benign presacral teratoma has been documented in at least three pediatric cases; all were 2- to 3-years-old girls. The importance of adherence to general rules of complete resection of a benign sacrococcygeal teratoma is well known: clear histologic safety margins, avoiding intraoperative tumor rupture and concomitant removal of the coccyx are key elements to minimize risk of recurrence. Until now, there have been ten cases of malignant teratoma reported in adults and six in children (all girls under five years of age) could represent malignant transformation of benign lesion or denovo malignancy [12,18].
Whereas constipation is the most common symptom and complication of the disease, CS may be associated with recurrent meningitis, due to rectomeningeal fistula, that carries a high mortality rate (can reach 56%) [19].
The treatment protocol of CS differs according to the clinical type presentation; so, it is imperative for the surgeons to decide the timing and the site that should be repaired first. Martucciello, et al. in 2004 have proposed the management strategy for CS. Their protocol stated that colostomy and neurosurgical intervention should be primarily performed in complete CS with a tethered cord. Plus, in cases with hind gut anomalies with intact neural tube, they indicated that primary Posterior Sacral Anorectoplasty (PSARP) should be firstly performed [13,9].
In case with a high anorectal atresia, multi-stage surgery is commonly planned involving initially colostomy and presacral tumor resection, followed by definitive operation for anal atresia as the further stage. In cases with a presacral nervous system tumor such as meningocele, colostomy should be performed first to avoid intraoperative soiling, even in presence of low anal atresia [2,13,14].
The rate of malignant transformation in CS may reach 1% of documented cases. Children with malignant transformation were under 5 years of age at diagnosis and occur 1 year post- excision of a macroscopically apparent benign tumor. Only one case that firstly presented with malignant teratoma was mentioned in the literature. Due to the rarity and easily missed diagnosis of CS, recurrence of a tumor should be kept in mind. Follow-up with serum tumor markers and MRI is recommended to detect any evolution of a pelvic mass [12].
In developed countries with pediatric surgical and fetomaternal centers, SCTs are diagnosed on routine prenatal ultrasound scan. Antenatal diagnosis often determines the eventual outcome of the pregnancy, the mode and the site of delivery of the affected babies. Other determining factors include age and weight of the fetus, the size of the tumor and its vascularity [20]. More vascular and large tumors detected on fetal scan or MRI would often require a caesarean section to avoid tumor rupture and significant bleeding leading to neonatal demise [21,22].
Conclusion
We report a case of incomplete Currarino’s syndrome comprising low imperforate anus and sacrococcygeal teratoma (with pre-sacral extension) but lacking the bony sacral anomaly component of the triad. Continuous follow up for recurrence of any presacral mass and evaluating the long-term outcomes are fundamental for proper management of cases.
Patient consent
No consent was obtained from the parents as the images & details presented in this article could not lead to identification of the patient.
Authorship
The authors attest that they meet the current ICMJE criteria for authorship.
- Ross AJ, Ruiz-Perez V, Wang Y, Hagan DM, Scherer S, et al. (1998) A homeobox gene, HLXB9, is the major locus for dominantly inherited sacral genesis. Nat Genet 20: 358-361. Link: https://bit.ly/34oaNLi
- Furuta S, Sato H, Hamano S, Kitagawa H (2015) Currarino syndrome associated with Hirschsprung’s disease: Case report and literature review. J Pediatr Surg Case Rep 3: 308-311. Link: https://bit.ly/2Xm41Ep
- Aly KA, Shoier M, Badrawy T (2006) Sacrococcygeal teratoma: a neonatal surgical problem. Ann Pediatr Surg 2: 106-111. Link: https://bit.ly/2UXYDph
- Spataru RI, Bratu N, Nisipasu CI, Iozsa DA (2014) Sacrococcygeal teratoma surgical treatment – a five year’s experience. J Pediatr 17: 42-45. Link: https://bit.ly/3eedDXB
- Kennedy RLJ (1926) An unusual rectal polyp: anterior sacral meningocele. Surg Gynecol Obstet 43: 803-804. Link: https://bit.ly/2yT3Zd3
- Currarino G, Coln D, Votteler T (1981) Triad of anorectal, sacral, and presacral anomalies. AJR Am J Roentgenol 137: 395-398. Link: https://bit.ly/3b0Owp9
- Isik N, Elmaci I, Gokben B, Balak N, Tosyali N (2010) Currarino triad surgical management and follow-up results of four cases. Pediatr Neurosurg 46: 110-119. Link: https://bit.ly/2JT4yWj
- Kassir R, Kaczmarek D (2014) A late-recognized Currarino syndrome in an adult revealed by an anal fistula. Int J Surg Case Rep 5: 240-242. Link: https://bit.ly/3eaza3n
- Jimbo T, Masumoto K, Urita Y, Sasaki T, Ono K, et al. (2015) Currarino syndrome with intramedullary spinal cord abscess related communication between the tethered cord and a presacral mass: A case report. J Ped Surg Case Rep 3: 432-435. Link: https://bit.ly/2RryQDM
- Morgenstern Isaak A, Bach Faig A, Martínez S, Martín-Nalda A, Vázquez Méndez E, et al. (2015) Recurrent meningitis due to anatomical defects: The bacteria indicate its origin. An Pediatr (Barc) 82: 388-396. Link: https://bit.ly/2XpaONB
- Gabler T, Westgarth-Taylor C, Loveland J (2016) An unusual cause for recurrent perianal sepsis in Currarino syndrome: Case report and review of the literature. J Ped Surg Case Rep 7: 16-19. Link: https://bit.ly/2V1FLWF
- Hage P, Kseib C, Adem C, Chouairy CJ, Matta R (2019) Atypical presentation of currarino syndrome: A case report. Int J Surg Case Rep 57: 102-105. Link: https://bit.ly/2y69nJ4
- Martucciello G, Torre M, Belloni E, Lerone M, Pini Parato A, et al. (2005) Currarino syndrome: proposal of a diagnostic and therapeutic protocol. J Pediatr Surg 39: 1305-1311. Link: https://bit.ly/3c1BPdU
- Cretolle C, Zerah M, Jaubert F, Samachi S, Revillon Y, et al. (2006) New clinical and therapeutic perspective in Currarino syndrome (study of 29 cases). J Pediatr Surg 41: 126-131. Link: https://bit.ly/2JTOXpp
- Dzienis-Koronkiewicz E, Debek W, Kuzmicz M (2013) Anal atresia and embryonic malignant teratoma. J Ped Surg Case Rep 1: 210-213. Link: https://bit.ly/2RvfKwG
- Emans PJ, Kootstra G, Marcelis CL, Beuls EA, van Heurn LW (2005) The Currarino triad: the variable expression. J Pediatr Surg 40: 1238-1242. Link: https://bit.ly/3b2iXva
- Gardner PA, Albright AL (2006) Like mother, like son: hereditary anterior sacral meningocele. Case report and review of the literature. J Neurosurg 104: 138-142. Link: https://bit.ly/2VmC5xD
- Little TA, Compson KE, Hall K, Murdoch MJ, Neas KR, et al. (2018) Currarino syndrome with two synchronous presacral teratomas. J Pediatr Surg Case Reports 36: 16-18. Link:
- Calleja Aguayo E, Estors Sastre B, Bragagnini Rodríguez P, Fustero de Miguel D, Martínez-Pardo NG, et al. (2015) Currarino triad: different forms of presentation. J Cir Pediatr 25: 155-158. Link: https://bit.ly/2VbIxHm
- Krishan S, Solanki R, Sethi SK (2004) Sacrococcygeal teratoma–role of ultrasound in antenatal diagnosis and management. JHK Coll Radiol 7: 35-39. Link: https://bit.ly/3b0PXE3
- Gabra HO, Jesudason EC, McDowell HP, Pizer BL, Losty PD (2006) Sacrococcygeal teratoma–a 25-year experience in a UK regional center. J Pediatr Surg 41: 1513-1516. Link: https://bit.ly/39UJRUF
- Cama JK, Nagra S (2013) Sacrococcygeal teratoma with an accessory limb an unusual presentation. J Ped Surg Case Rep 1: 42-44. Link: https://bit.ly/34pX7PT
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
