Archives of Clinical Gastroenterology
Primary Intestinal Lymphangiectasia diagnosed by Single-Balloon Enteroscopy
C Romero Mascarell1, IK Araujo1, I Ordás1, H Briceno2, D Monfort3, C Rodriguez-de Miguel1, A Ginés1, G Fernández-Esparrach1, M Cuatrecasas4,MT Forga5, J Llach1, and B González Suárez1*
2Department of Internal Medicine, Hospital d’Olot i Comarcal de la Garrotxa. Olot, Spain
3Department of Gastroenterology, Consorci Sanitari de Terrassa, Terrassa, Spain
4Pathology Department, Hospital Clínic de Barcelona, IDIBAPS and CIBEREHD, Barcelona, Spain
Cite this as
Mascarell CR, Araujo IK, Ordás I, Briceno H, Suárez BG, et al. (2018) Primary Intestinal Lymphangiectasia diagnosed by Single-Balloon Enteroscopy. Arch Clin Gastroenterol 4(3): 035-036. DOI: 10.17352/2455-2283.000057Primary intestinal lymphangiectasia (PIL) is a rare protein losing gastroenteropathy that usually affects children and teenagers. There are only a few cases described in the literature. The diagnosis is confirmed by the presence of intestinal lymphangiectasia based on endoscopic findings and histology.
We present a case of PIL diagnosed in a teenager patient, by Small Bowel Capsule Endoscopy (SBCE) and Single Balloon Enteroscopy (SBE).
Case Report
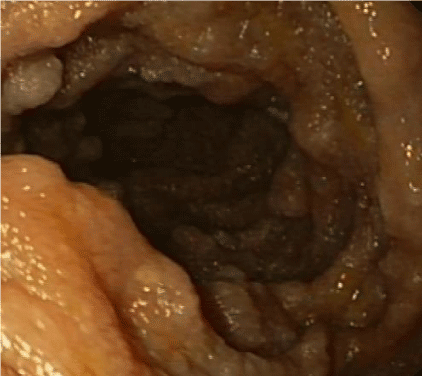
A 19- year-old male was admitted at a secondary care hospital for clinical signs of protein losing enteropathy, with diarrhea, hypoproteinemia, peripheral edema and low weight. Laboratory diagnoses showed decreased total proteins 4g/dL, albumins 2.5g/dL, zinc 55µg/dL (signs of malnutrition). The patient did not show any pathological or family history of interest. In an abdominal CT, diffuse wall thickening of jejunal and ileal loops was described. No lesions were identified in an upper GI tract endoscopy. A SBCE showed diffusely swollen mucosae, aphthaes, erosions and dilatation of lymphatic vessels affecting the entire small bowel mucosa. In distal jejunum, there was a polyposis-like pattern.
The patient was sent to a tertiary care hospital for a SBE. The mucosa of the small bowel had atrophy, edematous folds, loss of villi and dilated lymphatic vessels. In the jejunum, there were multiple lesions suggestive of pseudopolyps occupying the entire circumference. Biopsies were taken and the microbiological study ruled out Tropheryma whipplei infection (PCR). The histological analysis revealed villous widening, vascular congestion and lymphangiectasia without intraepithelial lymphocytosis. These signs were suggestive of primary intestinal lymphangiectasia (PIL).
Nutritional treatment was started with low long chain triglycerides and high protein diet supplements with medium chain triglyceride. Diarrhea and edemas improved and laboratory parameters normalized after three months of treatment Nowadays our patient is asymptomatic, under the same treatment and medical control every 6 months.
Discussion
PIL is a rare protein losing gastroenteropathy that usually affects children and teenagers. There are only a few cases described in the literature. It is caused by a congenital malformation or obstruction of intestinal lymphatic drainage, characterized by dilated lymphatic vessels of the gastrointestinal tract. When the pressure of lymphatic vessels increases, those dilate and rupture, resulting in leakage of lymph fluid into the bowel lumen that contains a great amount of protein, fat and lymphocytes. As a consequence, this causes hypoproteinemia, lymphocytopenia and decreased serum levels of immunoglobulin. Edema, diarrhea and ascites are the most common signs, but patients may also present chylothorax, pericarditis, lymphedema, fatigue, diarrhea, growth retardation and smaller physical constitution.
Laboratory findings include lymphopenia and decreased serum total protein and albumin along with decreased immunoglobulin levels including IgA, IgM, and especially IgG [1-3].
The diagnosis is confirmed by the presence of intestinal lymphangiectasia based on endoscopic findings and histology. Upper GI tract endoscopy and colonoscopy could be used to explore the duodenum or terminal ileum, but since intestinal lymphangiectasia often occurs at jejunum-ileum, the biopsies of duodenum or terminal ileum may be negative [4-9]. As a non-invasive procedure SBCE could be more useful to explore the entire small bowel mucosa. The typical endoscopic image is diffusely elongated, circumferential and polypoid mucosa covered with whitish enlarged villi. SBCE is very helpful, but the definite diagnosis is made by the histological examination obtained by BE, with the presence of dilated mucosal and submucosal lymphatic vessels.
In our case we were able to visualize the lesions in the entire small bowel by the SBCE. These findings guided us to perform a SBE and mucosal biopsies were taken to achieve the final diagnosis.
Treatment of PIL is not well defined. A low-fat diet with medium-chain triglyceride is the cornerstone of PIL treatment. Medium-chain triglycerides, unlike the long chain triglycerides, are absorbed into the portal circulation and can bypass the lymphatic system, avoiding the increase of lymphatic vessel pressure and the loss of protein. In severe cases, parenteral nutrition could be a complementary treatment.
In most patients, dietary treatment is permanently needed, because clinical and biochemical findings recur after treatment withdrawal [3,10,11].
Since the first description of the disease, a few cases of lymphoma have been reported suggesting its association. Patients developing lymphoma, do so after decades after the diagnosis. Treatment is surgery and postoperative chemotherapy or chemotherapy alone [12-15].
In conclusion, PIL is a rare disease where the clinical suspicion and an endoscopic approach are crucial for the diagnosis.
- Waldmann TA, STteinfeld JL, Dutcher TF, Davidson JD, Gordon RS Jr. (1961) The role of the gastrointestinal system in "idiopathic hypoproteinemia". Gastroenterology 41: 197-207. Link: https://goo.gl/CEPSto
- Vignes S, Bellanger J. (2008) Primary intestinal lymphangiectasia (Waldmann’s disease). Orphanet J Rare Dis 3: 5. Link: https://goo.gl/sPiBdU
- Ingle SB, Hinge Ingle CR. (2014) Primary intestinal lymphangiectasia: Minireview. World J Clin Cases 16; 2: 528-533. Link: https://goo.gl/vq1TG8
- Chamouard P, Nehme-Schuster H, Simler JM, Finck G, Baumann R, et al. (2006) Videocapsule endoscopy is useful for the diagnosis of intestinal lymphangiectasia. Dig Liver Dis. 38: 699-703. Link: https://goo.gl/s52vPv
- Triantafyllou K. (2010) Can we improve the diagnostic yield of small bowel video-capsule endoscopy? World J Gastrointest Endosc 16; 2: 143-146. Link: https://goo.gl/NZSEN7
- Ersoy O, Akin E,Demirezer A, Yilmaz E, Solakoglu T, et al. (2013) Evaluation of primary intessssssstinal lymphangiectasia by capsule endoscopy. Endoscopy 45(S 02): E61-E62. Link: https://goo.gl/XMdR1C
- Hirano A, Matsumoto T, Esaki M, Fujita K, Iida M. (2010) Intestinal lymphangiectasia presenting with duodeno-jejunal polyposis: enteroscopic findings. Endoscopy 42: E281-E282. Link: https://goo.gl/AugHpw
- Oh TG, Chung JW, Kim HM, Han SJ, Lee JS, et al. (2011) Primary intestinal lymphangiectasia diagnosed by capsule endoscopy and double balloon enteroscopy. World J Gastrointest Endosc 16; 3: 235-240. Link: https://goo.gl/kkT9kQ
- Lai Y, Yu T, Qiao XY, Zhao LN, Chen QK. Primary intestinal lymphangiectasia diagnosed by double-balloon enteroscopy and treated by medium-chain triglycerides: a case report. J Med Case Rep. 2013: 14; 7:19. Link: https://goo.gl/Pi3j1V
- Tift WL, Lloyd JK. (1975) Intestinal lymphangiectasia. Long-term results with MCT diet. Arch Dis Child. 1975; 50: 269-276. Link: https://goo.gl/vURdTM
- Muñoz Conde J, Gómez de Terreros I,Sánchez Ruiz F, Gavilán Carrasco F,et al. (1976) Idiopathic intestinal lymphangiectasis. Evolution with M.C.T. An Esp Pediatr 9: 438-446. Link: https://goo.gl/bC9GLb
- Laharie D, Degenne V, Laharie H, Cazorla S, Belleannee G, et al. (2005) Remission of protein-losing enteropathy after nodal lymphoma treatment in a patient with primary intestinal lymphangiectasia. Eur J Gastroenterol Hepatol 17: 1417-1419. Link: https://goo.gl/hyjA48
- Bouhnik Y, Etienney I, Nemeth J, Thevenot T, Lavergne-Slove A, et al. (2000) Very late onset small intestinal B cell lymphoma associated with primary intestinal lymphangiectasia and diffuse cutaneous warts. Gut 47: 296–300. Link: https://goo.gl/nDerva
- Gumà J, Rubió J, Masip C, Alvaro T, Borràs JL. (1998) Aggressive bowel lymphoma in a patient with intestinal lymphangiectasia and widespread viral warts. Ann Oncol 9: 1355-1356. Link: https://goo.gl/5NJdCh
- Patel KV, Goel RM, Wong T. (2013) Diffuse large B-cell lymphoma recurrence complicating primary intestinal lymphangiectasia. Clin Gastroenterol Hepatol 11: e86-87. Link: https://goo.gl/P6Kxth
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
