Annals of Circulation
Comparison of complication and success rates of perclose proglide device with surgical cut down in patients undergoing TAVI and TEVAR/EVAR Procedures
Jamal Moosavi1, Somaye Ahmadi2*, Parham Sadeghipour1, Ata Firoozi3, Bahram Mohebbi1, Omid Shafe4, Hooman Bakhshandeh Abkenar5 and Ahoura Salehi Nobandegani6
2MD, Fellowship of Interventional Cardiology, Rajaie Cardiovascular Medical & Research Center, Iran University of Medical Science, Tehran, Iran
3MD, Full Professor of Cardiology, Interventional Cardiologist, Rajaie Cardiovascular Medical & Research Center, Iran University of Medical Science, Tehran, Iran
4MD, Associate Professor of Cardiology, Interventional Cardiologist, Angiologist, Rajaie Cardiovascular Medical & Research Center, Iran University of Medical Science, Tehran, Iran
5Associate Professor of Epidemiology, Heart Research Center, Rajaie Cardiovascular Medical and Research Center, Iran University of Medical Science, Tehran, Iran
6MD, Rajaie Cardiovascular Medical and Research Center, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
Cite this as
Moosavi J, Ahmadi S, Sadeghipour P, Firoozi A, Mohebbi B, et al. (2022) Comparison of complication and success rates of perclose proglide device with surgical cut down in patients undergoing TAVI and TEVAR/EVAR Procedures. Ann Circ 7(1): 001-007. DOI: 10.17352/ac.000019Copyright
© 2022 Moosavi J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: The Common Femoral Artery (CFA) is the most frequently used peripheral artery for Trans-catheter aortic valve implantation (TAVI) and Endovascular Aortic Repair (EVAR)/ Thoracic Endovascular Aortic Repair (TEVAR) procedures. CFA access hemostasis could be obtained by manual compression, surgical cut-down, or using arteriotomy closure devices, including ProGlide.
Method: During a retrospective cohort study we compared ProGlide with surgical cut-down hemostasis in 225 patients who underwent TAVI or EVAR/TEVAR, including 290 access sites, during a 10 years period in terms of access site complications, procedure length and post-procedural hospitalization duration.
Results: The success rate of hemostasis was 100% in the PP device group and 98.3% in the SCD group. The mean Procedure length was significantly shorter in ProGlide device hemostasis and the mean post-procedural hospitalization length had a non-significant difference between the two groups. Access site complications occurred in 21.1% of the ProGlide group and 26% of the SCD group.
Conclusion: Perclose ProGlide device is safe and effective for access site closure in procedures that need large sheaths and it is non-inferior to standard surgical cut-down. Training, experience and careful application of the device have significant importance in ensuring successful hemostasis.
Background
The Common Femoral Artery (CFA) is the most frequently used peripheral artery for Trans-catheter Aortic Valve Implantation (TAVI) and Endovascular Aortic Repair (EVAR)/ Thoracic Endovascular Aortic Repair (TEVAR) procedures.
Initially, post-procedure hemostasis was often obtained by manual compression. this led to the patient being on complete bed rest for a period of time and having back pain and local pain-induced vasovagal reaction during compression [1].
After a while, femoral access for this procedure was achieved by Surgical Cut Down (SCD) of one or both groins [2,3].
Currently, the total percutaneous approach, using Arteriotomy Closure Devices (ACD), is being used increasingly, because of the resultant smaller sheath sizes, less invasive techniques, compared with the SCD approach, reduced operating time, less necessity for general and total in-room anesthesia, less post-operative pain, and quicker ambulation [4].
Nowadays, following the use of ACD, Patients feel less pain, hemostasis is achieved faster and the patients are ambulated and discharged earlier compared to manual compression and total percutaneous approach is associated with reduced operating time, need for general anesthesia, total in-room anesthesia, groin complications, postoperative pain, earlier ambulation and hospital discharge and so improve patient satisfaction as compared to SCD [1,2,5,6].
The Suture-mediated ACDs reduce the need for surgical arteriotomy and thus are associated with reduced morbidity and procedural cost [7]. ACDs are classified as either passive or active devices. Passive devices include hemostatic pads and compression devices. Active devices include those that are collagen-based, clip-based, or suture-based. Suture-mediated vascular closure devices, including Perclose ProGlide (PP) device, deploy sutures to achieve hemostasis with a knot made either by a built-in device mechanism or manually, which is advanced towards the puncture site to achieve closure of the arteriotomy [1].
PP device, is the second generation of suture-mediated vascular closure devices, with a 6 Fr profile containing three parts: a delivery system (consisting of a handle, plunger, a monofilament non-absorbable polypropylene suture, guide and sheath), a knot pusher and a suture trimmer. The suture is a single 3 - 0 monofilament non-inflammatory polypropylene, characterized by high tensile strength [8]. It is indicated to close the CFA access site during diagnostic or interventional catheterization procedures using 5 – 21 Fr sheaths (maximum outer diameter, 26 Fr). For up to 8F sheaths one PP device and For larger than 8 Fr, a minimum of two devices and the pre-close technique are required [9].
Herein, we performed a comparison between the PP device and SCD in terms of complications and duration of the procedure and hospitalization in patients selected for a TAVI or TEVAR/EVAR procedure.
Materials and methods
We retrospectively investigated Patients who underwent TAVI or EVAR/TEVAR procedures between 2011 and 2021 in our center and access site hemostasis had been provided by the PP device or SCD. We compared the patients in terms of access site complications, procedure length and post-procedural hospital stay duration.
The exclusion criteria were CFA diameter of lower than 6 millimeters, severe calcification of CFA or calcification of anterior wall of CFA (as detected in pre-procedural CT-angiography), pre-existing Iliac artery stenosis or occlusion, pre-existing scar at the inguinal region, psychotic disorder, BMI of more than 30 kilograms per square meter and the emergent procedures.
The procedure time is defined as the time from initiation of sedation, recorded by an anesthesiologist, to its end, the most accurate time had been recorded in the files.
During post-procedure hospitalization duration, all the patients had been visited and examined by an expert cardiologist via inspection, palpation and auscultation for access site complications on a daily basis and the suspicious ones underwent access site color Doppler and soft tissue ultrasonography. Also, the Complete Blood Count (CBC) of all patients was checked after the procedure and then in a daily pattern or if needed every 6 or 12 hours and any fall in Hemoglobin or Hematocrit was investigated. All the EVAR/TEVAR patients were undergone CT-angiography of the aorta, Iliac arteries, and femoral arteries 3 days after the procedure and were also visited and examined in the clinic one month later. As well, all the TAVI patients were visited and examined in the clinic one week and one month later.
We considered access site hemostasis success rate as the primary endpoint and our secondary endpoints were including:
- new onset lower extremity ischemia with or without requiring surgical or percutaneous intervention
- major bleeding (retroperitoneal hemorrhage, need for transfusion of 3 units or more pack cells, inguinal hematoma with a diameter of 5 centimeters or more)
- Minor bleeding (access site bleeding, need for transfusion of fewer than 3 units of pack cells, inguinal hematoma with a diameter of fewer than 5 centimeters)
- hematoma
- Pseudoaneurysm
- Arteriovenous fistula
- Iliac artery stenosis
- Localized access site infection treated with oral or parenteral antibiotics
- Procedure length (was defined as the time from the first skin incision to final closure measured by minutes)
- Hospitalization duration after the procedure (was defined as the time from sheath removal to actual physical discharge from the hospital expressed y days)
We defined successful hemostasis as successful achievement of index procedure ipsilateral access site hemostasis with percutaneous closure without the need for intravenous antibiotics, blood transfusion due to access site hemorrhage, or surgical intervention because of complications according to PEVAR study [10]. But unfortunately, device failure, described in Guohua Hu´s [11] study as failure to preload the sutures, suture rupture or tearing out, and insufficient tightening of the knots, was not documented in our retrograde data.
The results also were compared separately for TAVI or EVAR/TEVAR procedure and Confounding factors including Diabetes Mellitus (DM), Hypertension (HTN), renal failure (GFR: Glomerular Filtration Rate less than 60 mL/min/1.73m2), cigarette smoking, Chronic Obstructive Pulmonary Disease (COPD), Coronary Artery Disease (CAD), Cerebrovascular Disorders (CVD) and hypercholesterolemia (serum cholesterol more than 200 mg/dl).
Statistical analysis
Categorical data, including confounding factors, were compared using the Pearson chi-square test. p - value < 0.05 was considered a significant difference.
According to Kolmogorov-Smirnov Test, the age, procedure time and hospitalization duration have non-normally distributed so we expressed them by median statistics.
Binary variables were analyzed with the Logistic Regression test and we investigated the associations by using the Odds ratio (OR) parameter.
Also, we used the Mann-Whitney test for comparing procedure duration and post- rocedural hospitalization period.
Results
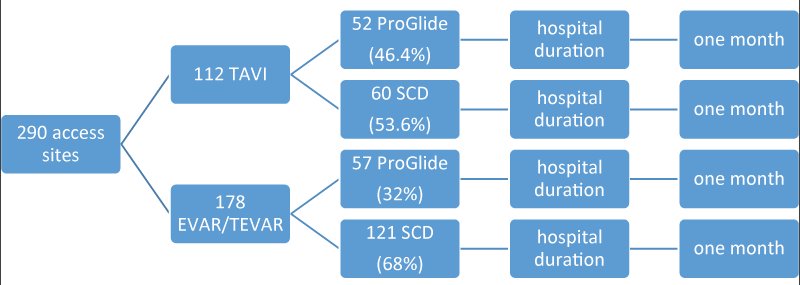
We analyzed a total of 225 patients, including 112 who underwent TAVI and 113 who underwent EVAR/TEVAR. 65 of EVAR patients had bilateral femoral accesses. So, in fact, our cases were 290 access sites, including 112 in TAVI patients and 178 in EVAR/TEVAR patients.
Access site hemostasis, defined as the successful achievement of access site hemostasis without the need for intravenous antibiotics, blood transfusion due to access site hemorrhage, or surgical intervention because of complications (according to the PEVAR study), was achieved with a PP device in 109 cases (37.5%) and SCD in 181 (62.5%). This statistic for the TAVI patients was 52 (46.4%) and 60 patients (53.6%) and for the EVAR/TEVAR group 57 (32%) and 121 (68%) respectively. These data have been shown in Figure 1.
Totally, 174 patients were men and 51 were women and of access sites, 237 (81.7%) were for men (84 in the PP device group and 153 in the SCD group) and 53 (18.3%) for women (25 in PP device group and 28 in SCD group), with the non-significant difference between the 2 groups.
The median statistic of age was 75 years in total, 76 years (68.5 - 80.5 years) for patients with SCD and 70 years (64.5 - 76 years) for patients with the ProGlide device. the PP closure group age was significantly lower than the SCD group. (p – value = 0.00)
Demographic data and confounding factors have been shown in Table 1.
The majority of confounding factors had non-significant differences between the two groups except cigarette smoking which significantly was more prevalent in the SCD group. (p – value = 0.006).
The success rate of hemostasis was 100% in the PP device group and 98.3% in the SCD group. That had a non-significant difference after statistical analysis. (p – value = 0.3)
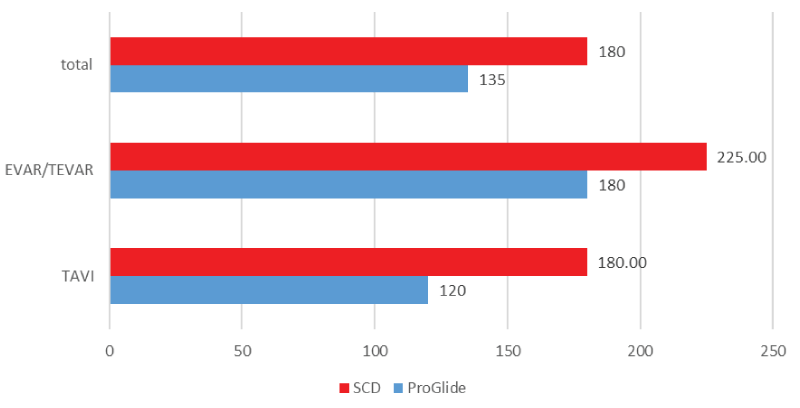
The mean Procedure length was significantly shorter in ProGlide device hemostasis, a total of 135 minutes (120 minutes - 180 minutes) in the ProGlide group and 180 minutes (180 minutes - 240 minutes) in the SCD group. (p = 0.00) This statistic for the patients who underwent TAVI was 120 minutes (105 minutes - 120 minutes) in the ProGlide group and 180 minutes (135 minutes - 180 minutes) in the SCD group (p = 0.00) and for the patients who underwent EVAR/TEVAR was 180 minutes (120 minutes - 240 minutes) and 225 minutes (180 minutes - 240 minutes). (p = 0.00)
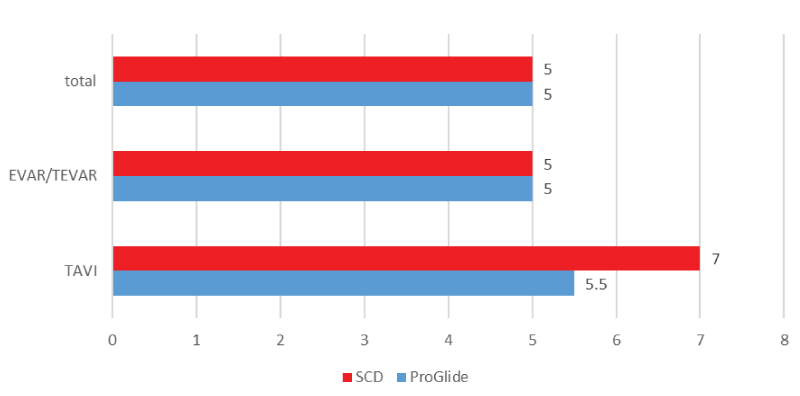
The mean post-procedural hospitalization length of all patients was 5 days (4 days - 7 days) in the ProGlide group and 5 days (4 days - 8 days) in the SCD group had a non-significant difference. (p = 0.1) This statistic for the patients who underwent TAVI was 5.5 days (4 days - 7 days) in the ProGlide group which was significantly shorter than the SCD group with a mean post-procedural hospitalization length of 7 days (5 days - 10 days). (p = 0.001) patients who underwent EVAR/TEVAR had no difference in terms of mean post-procedural hospitalization length between the PP device (5 days: 4 days - 7 days) and SCD (5 days: 4 days - 6 days). (p = 0.47)
The data of mean Procedure length and post-procedural hospitalization length has been summarized in Figures 1&2.
Generally, Access site complications occurred in 70 cases (24.1%) including 23 patients with ProGlide hemostasis (21.1%) and 47 patients with SCD hemostasis (26%). The difference between the two groups was non-significant (p – value = 0.3). These statistics for TAVI patients were 27 (24.1%), 8 (15.3%) and 19 (31.6%) and for EVAR/TEVAR patients were 43 (24.1%), 15 (26.3%) and 28 (23.1%) respectively.
During post-procedural hospitalization, routine physical examinations detected access site hematoma in 27 access sites (9.3%) and 7 cases (6.4%) were from the PP device group and 20 (11%) from the SCD group. (p = 0.18)
Nine patients (3.1%) suffered from major bleeding, including 3 patients (6.4%) of the ProGlide group and 6 patients (3.3%) of the SCD group. (P-value=0.7) just one patient of the PP device group suffered a retroperitoneal hemorrhage, manage conservatively and none needed a blood transfusion.
The difference in hematoma and major bleeding between the two groups was non-significant even after the elimination of the confounding effect of procedure type (TAVI or EVAR/TEVAR) and other confounding factors. (for hematoma p – value = 0.9, OR = 1.021, with 95% CI of 0.15 - 6.8, for major bleeding p – value = 0.7, OR = 0.5, with 95% CI of 0.027 - 12.6).
The incidence rate of minor bleeding was 45 access sites (15.5%) including 12 (11%) access sites in the ProGlide group and 33 (18.2%) in the SCD group. Although this value was higher in the SCD group, after statistical analysis, this difference was not significant. (p – value = 0.1) according to Logistic regression analysis for the elimination of confounding factors, this difference is yet non-significant but in men and hypertensive patients is borderline. (for men comparing women: p – value = 0.8, OR = 1.005, 95%CI of 0.96 - 1.04, for HTN: p – value = 0.06, OR = 2.07, 95% CI of 0.96 - 4.45).
A number of detected each pseudoaneurysm, arteriovenous fistula (AVF), and Iliac artery stenosis were detected in follow-up color-Doppler ultra-sonography, and CT-angiography was one (0.3% of all patients), all were in PP device group.
Ten patients (3.5%) during hospitalization had signs and/or symptoms of lower limb ischemia, 5 in the ProGlide group (4.6%) and 5 in the SCD group (2.8%). This difference however higher in the ProGlide group, was not significant. (p = 0.4) after Logistic regression analysis, we found that CAD has an association with this complication. (p = 0.05, OR = 5.07, 95% CI of 0.93 - 27.4).
Access site infection occurred in 4 cases (1.4%) including one of the ProGlide group (0.9%) that was resolved with oral antibiotics and the patient had been discharged after 5 days. Also, 3 of the patients who underwent SCD as hemostatic technique (1.7%) suffered access site infection, and all of them needed long-term at least 11 days of treatment with intravenous antibiotics. The difference was non-significant. (p = 0.6)
The data on comparison between the two groups have been summarized in Figure 3.
Discussion
The Perclose ProGlide provides effective hemostasis in patients requiring extensive arterial opening such as the TAVI and the EVAR/TEVAR procedures and improves the early mobilization of patients and reduces the length of hospital stay. Therefore, it is increasingly used during these procedures [1].
Careful patient and device selection and operating procedure are paramount to achieving successful outcomes [9].
In this study, we present a comparison between the outcome of using a PP device versus SCD for Femoral artery hemostasis in the patients who underwent TAVI or EVAR/TEVAR procedures in our center. Our study consisted of 225 patients, including 290 access sites, 112 for TAVI, and 178 for EVAR/TEVAR patients. Access site closure in 37.5% of patients was achieved with a PP device and in 62.5% by SCD. Distribution of sex and confounding factors including DM, HTN, hypercholesterolemia, COPD, CAD, CVD and renal failure were similar between the two groups except cigarette smoking significantly was more prevalent in the SCD group. Also, the median age of the PP device group was 6 years lower than the SCD group which was a significant difference. The success rate of hemostasis had no significant difference between the two groups (100% in the PP group and 98.3% in the SCD group). The mean Procedural length was significantly shorter in PP device hemostasis (135 minutes in the ProGlide group versus 180 minutes in the SCD group). The mean length of hospital stay had a non-significant difference between the two groups totally, but the TAVI patients were significantly shorter in the PP device group. We had access site complications in 70 of 290 cases (24.1%) including 23 (24.1%) in the PP device group and 47 (24.1%) in the SCD group with a non-significant difference between the two groups.
Peter R Nelson, et al. [10] during the PEVAR trial, analyzed 151 patients undergone EVAR/TEVAR Between 2010 and 2012 and compared Perclose devices (n = 101: ProGlide (n = 50) or Prostar XL (n = 51)) with standard SCD (n = 50). Baseline characteristics were similar among groups. Perclose ProGlide and Prostar XL use were associated with significantly shorter times to hemostasis and procedure completion and favorable trends in blood loss, groin pain, and overall quality of life.
Early studies mentioned a long learning curve and many exclusion criteria, such as calcified arteries and morbid obesity. SCD equally remains a challenge in these patients [8,9,12].
MingChen, et al. [9] in a retrospective study, used the PP device to achieve vascular access site closure in 458 patients in 602 access sites undergoing TAVI, TEVAR, or EVAR procedures. The ProGlide failure occurred in 7.6% of cases. They acknowledged that Factors such as morbid obesity, history of PAD, the presence of CFA calcification, the depth of the skin puncture site and sheath size are significantly associated with ProGlide failure [9]. so we excluded the patients with these factors from our study. So finally We involved 225 patients, including 174 men and 51 women in our study including 290 access sites.
The pre-close technique with the ProGlide device is associated with a technical success rate of 92% and a failure rate of about 2% – 8% [1,8,10]. we achieved 100% successful hemostasis using the PP device for access site hemostasis, defined as successful achievement of index procedure ipsilateral access site hemostasis with percutaneous closure without surgical intervention, need to blood transfusion or intravenous antibiotics in PEVAR study [8].
The incidence of PP device complications was found to be up to 20% in the literature including hematoma, bleeding, pseudoaneurysm, AVF, retroperitoneal hemorrhage, thrombosis, infection and femoral artery stenosis or occlusion [6]. in our study, Access site complications occurred in 23 of 109 cases (21.1%) including 19 of PP device group hemostasis (17.4%) and 38 patients with SCD hemostasis (21%). These statistics for TAVI patients were 23 (20.5%), 6 (11.5%) and 17 (28.3%) and for EVAR/TEVAR patients were 34 (19.1%), 13 (22.8%) and 21 (17.3%) respectively.
Dominique B Buck, et al. [2] identified 4112 patients with abdominal aortic aneurysms undergoing EVAR, using either ACD (n = 1108, 27%) or SCD (n = 3004, 73%) as access site closure technique. ACD group had shorter operative time (mean, 135 vs 152 minutes), shorter length of stay (median, one day vs 2 days), and fewer access site complications (2.1% vs 1.0%). Also, Vahid Etezadi, et al. [6] reviewed retrospectively 445 patients undergoing aortic aneurysm repair. Such a complication rate after successful vascular closure with ACD (9.4%) was significantly less than SCD (19.4%). When failed cases were considered as a complication, the complication rate was not different between the two groups, similar to our study and literature.
Median procedural time in EVAR/TEVAR patients in our study was 180 minutes in the PP device group and 225 minutes in the SCD group. the total times compared to Buck’s study, in our study were longer but similarly, procedure time was with a significant difference, shorter in the PP device group. (p = 0.00) longer procedure time in our study probably is due to a combination of TEVAR/EVER patients, unlike Buck´s study that only involves the EVAR patients. Besides, in our study, the procedure time was defined as the time from initiation of sedation, recorded by an anesthesiologist, to its end, the most accurate time had been recorded in the files, but in Buck´s study defined as the first skin break to complete skin closure. The median hospital length of stay among PP device-assisted hemostasis in EVAR/TEVAR patients in our study was 5 days (4 - 7) which was similar to that of the SCD group. Longer hospitalization in our patients was the majority due to fever of unknown origin, UTI, endoleak and other complications of the procedure and only 10 (3.4%) were due to access site complications.
A Systematic Review [4] and Meta-analysis of 17 Randomised Clinical Trials and Cohort Studies, contained 7889 access sites, designed to assess the differences between ACDs and SCD of the CFA. The majority of patients were male (78%) as in our study. Reported patient comorbidity consisted of smoking, DM, HTN, CAD and renal impairment. These comorbidities were equally divided between the ACD and SCD groups. Table 1 compares these comorbidities in our study with the systematic review, showing that our statistics are between these ranges.
This systematic review revealed a total of 8% of complications were seen in SCD compared with 6.8% after ACD use in EVAR and TAVI patients. ACD was associated with fewer surgical site infections (OR 0.38, 95% CI 0.23 – 0.63). pseudoaneurysms were significantly more common in the ACD group (OR 3.8, 95% CI 1.6 – 9.4), The only significant advantage of SCD, is probably because the repair is performed under direct vision. The randomized controlled trials are not signed in favor of ACDs in terms of seroma formation. There were no differences regarding post-operative hematoma or post-operative stenosis or occlusion of the CFA between the two groups, just as in our study. This study used mean statistics for the duration of the procedure and hospitalization but we used median. However, the difference between the ACD and SCD groups was not significant neither regarding procedure duration nor hospitalization length in this study. As well in our study the mean post-procedural hospitalization length of all patients and in EVAR/TEVAR patients had a non-significant difference between the two hemostatic techniques but for the patients who underwent TAVI in the PP group was significantly shorter than SCD group also the mean Procedural length in all patients and separately in two procedures was significantly shorter in PP device hemostasis.
Gunduz Durmus, et al. [1] analyzed 74 patients, including 58 undergoing TAVI and 16 undergoing EVAR, who received ProGlide for access site hemostasis. two (3.4%) Of the TAVI patients had access site bleeding complications and of the EVAR patients three (18.8%). lower success and higher complication rate in the EVAR group is due to the underlying diffuse aortic wall pathology.
Ahmet A Sahin, et al. [13] compared retrospectively a total of 96 patients with type III aortic dissection who underwent TEVAR: in 56 hemostasis was done with a PP device and 40 with SCD.
There was no significant difference between the two groups in terms of complications and technical success. The overall success rate for the PP device was 94.6% and for SCD 100% for the surgical approach. Access site complications occurred in three of the PP groups and four in the SCD group.
Kyriakos Oikonomou, et al. [12] during a retrospective study analyzed 263 patients, using 376 puncture sites, undergoing EVAR or TEVAR procedures between April 2017 and June 2021, whose hemostasis was done with a PP device. The primary technical success rate was 93.1%. Postoperative complications occurred in 13 cases (3.5%), 2 of which required surgical reintervention.
Dennis Eckner, et al. [14] observed 787 patients undergoing a TAVR-Procedure between 2013 and 2019 retrospectively. Of those, in 338 patients hemostasis was performed with SCD and in 449 with the PP device. 2.8% of their patients died, including 3.8% of the SCD group and 2.2% of the PP group, with no significant difference between them. Also, major vascular complications or bleeding were not significantly different in either group (SCD group 5.3% versus PP group 5.1%).
Sven Ross Mathisen, et al. [15] in a case series investigated 434 elective and acute EVAR procedures between May 2011 and July 2017. Of the 837 groins that had access closure with the ProGlide device, Primary technical success was achieved in 68.1%, secondary in 96.9%, and 31 groin complications (3.7%) were registered during 30-day follow-up, 17 required additional treatment. Total mortality was 2.8%. None were related to the access site.
We detected in our ProGlide group, bleeding complications in 9 of EVAR/TEVAR patients (6.3%) and 6 of TAVI patients (8.6%). A low number of patients is the reason for the lower complication rate comparing our study.
In our study, the difference between the two groups regarding major bleeding, hematoma, access site infection, and lower limb ischemia was non-significant (p - value = 0.18, 0.7, 0.6 and 0.4 respectively). This was non-significant even after the elimination of the confounding effect of procedure type (TAVI or EVAR/TEVAR) and other confounding factors for major bleeding, and hematoma. (for hematoma p = 0.9, OR = 1.021, with 95% CI of 0.15 - 6.8, for major bleeding p = 0.7, OR = 0.5, with 95% CI of 0.027 - 12.6). after Logistic regression analysis, we found that CAD has an association with lower limb ischemia. (p = 0.05, OR = 5.07, 95% CI of 0.93 - 27.4).
The incidence rate of minor bleeding was higher in the SCD group, but after statistical analysis, this difference was not significant. (p = 0.1) and yet non-significant despite the elimination of confounding factors, but in men and hypertensive patients was borderline.
A number of detected pseudoaneurysms, arteriovenous fistula (AVF), and Iliac artery stenosis detected in follow-up color-Doppler ultra-sonography and CT-angiography each was one (0.3% of all patients), in our research, all were in ProGlide group.
Conclusions
ProGlide-assisted hemostasis in patients who undergo procedures that required wide femoral access extension, is safe and effective, with low access-related complications and it is non-inferior to SCD. The learning curve, experience and careful application of this device have major significance in success rate and decrease complications and outcomes.
Declarations
Availability of data and materials: The authors confirm that the data supporting the findings of this study are available within the article.
Competing interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Authors’ contributions
Jamal Moosavi: Provided the conception and design of the procedure and performed the procedure.
Somaye Ahmadi: Supplied the acquisition of data and wrote the manuscript.
Ata Firouzi, Parham Sadeghipour, Omid Shafe and Bahram Mohebbi: Were involved in the procedures.
Hooman Bakhshandeh Abkenar: Performed the statistical analysis
Ahoura Salehi Nobandegani: Collected the data
Author,s information
Jamal Moosavi, MD, Associate Professor of Cardiology, Interventional Cardiologist, Rajaie Cardiovascular Medical & Research Center, Iran University of Medical Science, Tehran, Iran, Email: drjamalmoosavi@gmail.com. ORCID: 0000000159641724.
Somaye Ahmadi, MD, Fellowship of Interventional Cardiology, Rajaie Cardiovascular Medical & Research Center, Iran University of Medical Science, Tehran, Iran, Email: dr_s_ahmadi@yahoo.com. ORCID: 0000000179031958.
Parham Sadeghipour, MD, Associate Professor of Cardiology, Interventional cardiologist, Rajaie Cardiovascular Medical & Research Center, Iran University of Medical Science, Tehran, Iran, Email: psadeghipour@hotmail.com.
Ata Firouzi, MD, Full Professor of Cardiology, Interventional Cardiologist, Rajaie Cardiovascular Medical & Research Center, Iran University of Medical Science, Tehran, Iran, Email: atafirouzi@yahoo.com.
Bahram Mohebi, MD, Associate Professor of Cardiology, Interventional Cardiologist, Rajaie Cardiovascular Medical & Research Center, Iran University of Medical Science, Tehran, Iran, Email: roodbar@yahoo.com.
Omid Shafe, MD, Associate Professor of Cardiology, Interventional Cardiologist, Angiologist, Rajaie Cardiovascular Medical & Research Center, Iran University of Medical Science, Tehran, Iran, Email: omidshafe@hotmail.com.
Hooman Bakhshandeh Abkenar, Associate Professor of Epidemiology, Heart Research Center
Rajaie Cardiovascular Medical and Research Center, Iran University of Medical Science, Tehran, Iran, Email: bakhshandeh@rhc.ac.ir.
Ahoura Salehi Nobandegani, MD, Rajaie Cardiovascular Medical and Research Center, School of Medicine, Iran University of Medical Sciences, Tehran, Iran. E-mail: Ahoura.salehi@yahoo.com.
- Durmuş G, Belen E, Bayyiğit A, Can MM. Comparison of Complication and Success Rates of ProGlide Closure Device in Patients Undergoing TAVI and Endovascular Aneurysm Repair. Biomed Res Int. 2018 Aug 9;2018:2687862. doi: 10.1155/2018/2687862. PMID: 30175119; PMCID: PMC6106714.
- Buck DB, Karthaus EG, Soden PA, Ultee KH, van Herwaarden JA, Moll FL, Schermerhorn ML. Percutaneous versus femoral cutdown access for endovascular aneurysm repair. J Vasc Surg. 2015 Jul;62(1):16-21. doi: 10.1016/j.jvs.2015.01.058. Epub 2015 Mar 28. PMID: 25827969; PMCID: PMC4484310.
- Nakamura M, Chakravarty T, Jilaihawi H, Doctor N, Dohad S, Fontana G, Cheng W, Makkar RR. Complete percutaneous approach for arterial access in transfemoral transcatheter aortic valve replacement: a comparison with surgical cut-down and closure. Catheter Cardiovasc Interv. 2014 Aug 1;84(2):293-300. doi: 10.1002/ccd.25130. Epub 2014 Apr 30. PMID: 23873857.
- Vierhout BP, Pol RA, El Moumni M, Zeebregts CJ. Editor's Choice - Arteriotomy Closure Devices in EVAR, TEVAR, and TAVR: A Systematic Review and Meta-analysis of Randomised Clinical Trials and Cohort Studies. Eur J Vasc Endovasc Surg. 2017 Jul;54(1):104-115. doi: 10.1016/j.ejvs.2017.03.015. Epub 2017 Apr 21. PMID: 28438400.
- Del Prete A, Della Rocca DG, Calcagno S, Di Pietro R, Del Prete G, Biondi-Zoccai G, Raponi M, Scappaticci M, Di Matteo A, Natale A, Versaci F. Perclose Proglide™ for vascular closure. Future Cardiol. 2021 Mar;17(2):269-282. doi: 10.2217/fca-2020-0065. Epub 2020 Sep 11. PMID: 32915065.
- Youn YJ, Khalid S, Azrin M, Lee J. Stenosis Caused by Suture-Mediated Vascular Closure Device in an Angiographic Normal Common Femoral Artery: Its Mechanism and Management. Vasc Endovascular Surg. 2019 Jan;53(1):58-61. doi: 10.1177/1538574418791883. Epub 2018 Aug 9. PMID: 30092748.
- Metcalfe MJ, Brownrigg JR, Black SA, Loosemore T, Loftus IM, Thompson MM. Unselected percutaneous access with large vessel closure for endovascular aortic surgery: experience and predictors of technical success. Eur J Vasc Endovasc Surg. 2012 Apr;43(4):378-81. doi: 10.1016/j.ejvs.2011.12.025. Epub 2012 Jan 18. PMID: 22261486.
- Saadi EK, Saadi M, Saadi R, Tagliari AP, Mastella B. Totally Percutaneous Access Using Perclose Proglide for Endovascular Treatment of Aortic Diseases. Braz J Cardiovasc Surg. 2017 Jan-Feb;32(1):43-48. doi: 10.21470/1678-9741-2016-0065. PMID: 28423129; PMCID: PMC5382908.
- Chen IM, Lee TH, Chen PL, Shih CC, Chang HH. Factors in ProGlide® Vascular Closure Failure in Sheath Arteriotomies Greater than 16 French. Eur J Vasc Endovasc Surg. 2019 Oct;58(4):615-622. doi: 10.1016/j.ejvs.2019.03.037. Epub 2019 Sep 6. PMID: 31500989.
- Nelson PR, Kracjer Z, Kansal N, Rao V, Bianchi C, Hashemi H, Jones P, Bacharach JM. A multicenter, randomized, controlled trial of totally percutaneous access versus open femoral exposure for endovascular aortic aneurysm repair (the PEVAR trial). J Vasc Surg. 2014 May;59(5):1181-93. doi: 10.1016/j.jvs.2013.10.101. Epub 2014 Jan 17. PMID: 24440678.
- Hu G, Chen B, Fu W, Xu X, Guo D, Jiang J, Yang J, Wang Y. Predictors and treatments of Proglide-related complications in percutaneous endovascular aortic repair. PLoS One. 2015 Apr 22;10(4):e0123739. doi: 10.1371/journal.pone.0123739. PMID: 25901610; PMCID: PMC4406497.
- Oikonomou K, Kvataia A, Pfister K, Zygouridou E, Betz T, Schierling W, Sachsamanis G. Percutaneous Approach in Endovascular Aortic Procedures Using a Suture-Mediated Closure Device. J Clin Med. 2022 Nov 10;11(22):6660. doi: 10.3390/jcm11226660. PMID: 36431136; PMCID: PMC9695263.
- Sahin AA, Guner A, Demir AR, Uzun N, Onan B, Topel C, Çelik Ö. Comparison between PeRcutanEous and surgical femoral aCcess for endovascuLar aOrtic repair in patientS with typE III aortic Dissection (PRECLOSE Trial). Vascular. 2021 Aug;29(4):616-623. doi: 10.1177/1708538120965310. Epub 2020 Oct 14. PMID: 33054676.
- Eckner D, Pollari F, Santarpino G, Jessl J, Schwab J, Martinovic K, Mair H, Pauschinger M, Fischlein T, Vogt F. Comparison between Surgical Access and Percutaneous Closure Device in 787 Patients Undergoing Transcatheter Aortic Valve Replacement. J Clin Med. 2021 Mar 24;10(7):1344. doi: 10.3390/jcm10071344. PMID: 33805069; PMCID: PMC8037566.
- Mathisen SR, Nilsson KF, Larzon T. A Single Center Study of ProGlide Used for Closure of Large-Bore Puncture Holes After EVAR for AAA. Vasc Endovascular Surg. 2021 Nov;55(8):798-803. doi: 10.1177/15385744211022654. Epub 2021 Jun 9. PMID: 34105422.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
