Biomed Sci Eng
3D Bioprinting: An attractive alternative to traditional organ transplantation
Darakhshanda Iram1, Rafi a Riaz1 and Rana Khalid Iqbal1*
Cite this as
Iram D, Riaz RA, Iqbal RK (2019) 3D Bioprinting: An attractive alternative to traditional organ transplantation. Biomed Sci Eng 5(1): 007-018. DOI: 10.17352/abse.0000123D bioprinting is computer-aided technology used to generate 3D models of organs. Employing this technique, organ and tissues are generated according to the patient body. 3D structures are formed by the deposition of bioink. This bioink can be natural or synthetic bionink. For in vitro implantation, the tissue is first incubated in a bioreactor, however, in in vivo there is no prerequisite incubation required, rather cells are directly implanted. Bioprinting consists of various steps involving imaging, design approach, choice of material, cell selection and printing of tissue construct.3D bioprinting has two main approaches, i.e. cellular and a-cellular. Cellular bioprinting can be inkjet based, stereolithography based, laser induced forward transfer (LIFT) and extrusion based. Acellular bioprinting is extrusion based and laser based. Tissues of various organs are formed using 3D bioprinting involving blood vessels, bone, cartilage, heart, kidneys, and that of the skin and neurons. However, bioprinting of micro organs and the selection of suitable bioink is a difficult task. Bioprinting has various limitations that lead to the development of 4D bioprinting. This review paper will help you to understand the basic technique of 3D bioprinting, its application, limitations and new advancements that help to enhance the efficacy of this technique.
Abbreviations
3D: Three-Dimensional; CAD: Computer-Aided Design; LOM: Laminated Object Manufacturing; SLS: Selective Laser Sintering; FDM: Fused Deposition Modeling; MJM: Multi Jet Modeling; DLP: Digital Light Processing; SLA: Stereolithography; 4D: Four Dimensional; ECM: Extracellular Matrix; CT: Computed Tomography; MRI: Magnetic Resonance Imaging; TGF-β: Transforming Growth Factors Beta; RGD: Arginine-Glycine-Aspartic Acid; HA: Hyaluronic Acid; UV: Ultra Violet; PCL: Polycaprolactone; PEG: Polyethylene Glycol; PLGA: Poly Lactic-Co-Glycolic Acid; PLA: Poly Lactic Acid; PGA: Poly Glycolic Acid; PHEMA: Poly Hydroxyethyl Methacrylate; PAB: Pressure-Assisted Bioprinting; GFs: Growth Factors; DNA: Deoxyribo Nucleic Acid; LIFT: Laser-Induced Forward Transfer; 2D: Two Dimensional; DLP: Digital Light Processing; SLS: Selective Laser Sintering, SHS: Selective Heat Sintering; CVD: Cardiovascular Diseases; GelMA: Gelatin-Methacryloyl; HUVECs: Human Umbilical Vein Endothelial Cells; PU: Polyurethane; β-TCP: β-Tricalcium Phosphate; MSC: Mesenchymal Stem Cell; ASC Adipose Derived Stromal Stem Cell; CNS: Central Nervous System; SCs: Schwann Cells; RGC: Retinal Ganglion Cells; CLIP: Continuous Liquid Interference Production
Introduction
3-D bioprinting is a modern technology that is used in tissue engineering in order to develop tissues and organs similar to native ones [1]. Three-dimensional bioprinting technique was first introduced by Charles W. Hull in 1986 [2]. It is an additive manufacturing process, seems somehow attractive and thus attained a lot of public attention. Additive manufacturing help to generate a model. For end stage organ diseases, organ transplantation is the only cure. The availability of a donor and the organ match is a problem for organ transplantation, so the alternative is 3D bioprinting [3]. Previous to 3D bioprinting, the tissue bio fabrication method was used. In the traditional method of tissue fabrication, cells are first cultured to allow them to expand as a monolayer. A porous scaffold is formed prior to culturing and then cultured cells are seeded into the scaffold [4]. The scaffold is a very important structure for tissue engineering applications because it provides support and space for the growth of cells [5]. The cells applied on the scaffold must migrate into the scaffold, therefore scaffold must be highly porous and also safe for seeded cells. The scaffold that is employed should be degradable. A bioreactor is also required to give an environment similar to that of in vivo. The thin tissues can survive in the host because nutrients can easily diffuse through thin tissues. However, when the size of the engineered tissues increases above the range of 400 µm, diffusion of oxygen (O2) cannot occur at the required rate. In such situations, functional vasculature must be enabled to supply O2 and nutrients to the cells. Moreover, conventional tissue engineering fails to regenerate thick and complex tissues such as that of liver, kidney and heart [4]. Compared to the conventional fabrication method, 3-D bioprinting is more efficient [6]. In 3D bioprinting, solid objects are made using the technology of computer-aided design employing 3D software. This technique is associated with the formation of 3D structures using metals. Additionally, 3D equipment consists of 3D modeling software, a computer, bioprinting materials and x, y and z-axis machine. The 3D equipment is connected to the computer in order to form a 3D scaffold structure. 3D images are formed using CAD. The methods of 3D bioprinting consist of laminated object manufacturing (LOM), selective laser sintering (SLS), fused deposition modeling (FDM), multi jet modeling (MJM), digital light processing (DLP) and stereolithography (SLA). Liquid polymer resin and ultraviolet lasers are used in SLA and DLP in order to create 3D images. Material is fed through a small diameter nozzle in the material inject process in MJM method. In MJM, print head technology is utilized for the layer by layer deposition of photocurable plastic resin or casting wax material. However, thermoplastic filaments are used as bioprinting material in FDM bioprinting. Small particles such as polymers and ceramics employing high power laser are used to create 3D images in SLS. In LOM 3D models are created using polymers, plastics and metals. Among these 3D bioprinting methods, SLA, DLP and FDM are used in the tissue engineering process [2]. The material which is deposited layer by layer for the formation of cells is known as “bioink”. It is an ink formulation that dictates the printing of living cells. However, various biomaterials are not suitable for the formation of 3D printing of the living cells [7]. In 3D bioprinting, tissues are incubated in bioreactor in vitro, before their implantation in the human body. Cells can also be directly imprinted in living cells under in invivo conditions in which human body act as bioreactor [8]. The additive manufacturing technique is design dependent for the formation of the scaffold. According to patient specification, the size, geometry and porosity of the scaffold can be controlled. This technique is highly reproducible [6]. Some tissues possess unique functions that can be obtained by dynamic changes in tissue conformations. In the heart and brain, the electrical signaling is very important e.g. in heart pumping and in the signaling of brain peristaltic movements. Therefore the 3D structure is not sufficient for biomedicine [9]. So, next is the era of 4D bioprinting based on 3D bioprinting which fulfill the need of stimulus responsive geometry.In this review, we will present 3D bio printing approaches, techniques, medical applications of 3D bio printing and the next era of 4D bioprinting.
3D bioprinting approaches
In 3D bioprinting three approaches are included which are biomimicry, autonomous self-assembly, tissue building blocks.
Biomimicry
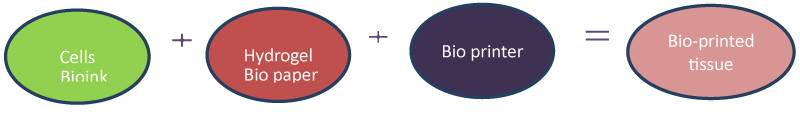
Biological engineering has helped to enhance the efficacy of many fields. Many technological problems are solved by biological engineering for example it helped in nanotechnology, cell culture methods [10] and research materials [11]. 3D bioprinting application to biomimicry involves the formation of identical, cellular and extracellular components of tissues [12]. In biomimicking, physiologically identical biomaterials are manufactured. After the formation of a biologically similar component, its replication is done on a micro scale. Thus complete understanding of functional and supporting cell type, soluble and insoluble gradient factors and the biological forces involved in micro-environment, is needed. With a complete understanding of all these aspects, this approach can be successfully applied in imaging, biomaterials engineering, biophysics, cell biology and medicine. Components required for 3D bioprinting of tissues are given in (Figure 1) [13].
Autonoumous self-assembly
Embryonic organ development can be used as a guide to replicate biological tissues. The early cells that are produced during the development of tissues produce their own ECM components, cell signaling and pattering that yield desired biological function [14] [15]. This approach use self-assembly of cellular spheroids for the fusion of cellular organizations to construct developing tissues. In this approach, the cell is considered as the primary driver of histogenesis that directs the composition, localization and structural and functional properties of tissues [16,17]. Complete understanding of tissue genesis, organogenesis and the ability of tissue to drive embryonic mechanism is required for this approach to carry on.
Tissue building blocks
The concept of tissue building blocks is related to both above mentioned strategies. Smaller functional building blocks also known as mini tissues form organs and tissues. These tissues can be defined as the smallest structural and functional building block of any organ such as nephron in the kidney. These mini tissues are combined together by rational design or self-assembly. Two major strategies are used for this approach. First, cell spheres are self-assembled to macro tissues. [18,19]. Second, tissue units of high resolution reproduction are designed and allowed to self-assemble to form functional macro tissues. These approaches include the formation of vascular building blocks in order to form branched vascular networks. This is helpful in the in vitro model of diseases for the screening of drugs and vaccines on “organs on chip” [20-22]. Above strategies combine to form a complex 3D structure with complete, structural, functional and mechanical properties. The main steps involved in bioprinting are imaging, design approach, the choice of material, cell selection and printing of tissue construct [23], given in (Table 1).
Bioprinting requirements
Bioprinting is the process which refers to the conversion of liquid biomaterial after printing in layer-by-layer fashion into solid 3D structures [24]. This conversion from solid to gel or phase transition procedure is the main principle of 3D bioprinting [24]. In addition, pre-polymer solution and cells are main components of bioinks used in the bioprinting technique [25]. Bioink actually defines the liquid solution containing tissue sphenoids or particular cell suspensions [26]. 3D bioprinting most extensively utilizes organic solutions, including alginate hydrogels, collagen and hyaluronic acid as its biomaterials [26]. Additionally, various synthetic biomaterials such as PCL, are characterized by their ability to provide mechanical support to the cell-laden tissue constructs [26]. However, nanocellulose materials, due to their high water content, mechanical strength and shear thinning properties have proved to be a good material for the fabrication of 3D constructs [26]. 3D bioprinting technique involves various bioinks which should exhibit various important properties and characteristics including printability, mechanical properties, biodegradation in the controlled environment and non-toxicity to cells [1].
Natural bioinks
Hydrogels are extensively used as the natural biomaterials which usually print by enclosing and subsequent printing living cells due to its analogous nature to native tissue microenvironment [27]. Various cellular behaviors such as migration, proliferation, differentiation and maturation are also controlled by tissue-specific, biochemical and physical stimuli delivered by these hydrogels [28]. Natural hydrogels are made up of various chemical and physical components which depends greatly upon target tissue and cell types [29]. Moreover, the cellular matrix released by various cell types usually comprises of tissue-specific growth factors and chemokines including transforming growth factors beta (TGF-β), epidermal growth factor, insulin-like growth factor and matrix metallo proteinase [30,31]. However, there are few shortcomings of natural hydrogels, the major one is batch-to-batch variability [32,33].
Alginate is the algae driven anionic polysaccharide which is made up of two repeating monosaccharides (i.e. L-guluronic and D-mannuronic acids) which form a hydrogel by exploiting multivalent cations such as Ca2+, Ba2+, and Fe 3+ [27]. Perfect biocompatibility and simple and fast gelation are the few advantages that make alginate suitable for the fabrication of 3D tissue/organ [27,34].
Collagen is another natural polymer which is composed of the large quantity of glycine, proline and hydroxyproline residues. Collagen is a part of various extracellular matrix (ECM) and is involved in physiological interactions between cells and ECM. Simple cross-linking via thermo-responsible gelation under physiological conditions is the main advantage of collagen based bioink.
Gelatin is a form of collagen which is denatured and has its application in the food, pharmaceutical and cosmetic industry as a gelling agent. Cell adhesion is attained via integrin receptors due to the presence of proteins including fibronectin, vimentin, vitronectin, and RGD peptides.
Fibrin is composed of fibrinogen and thrombin interactions and these interactions are referred to blood coagulation mechanism. It acts as a surgical glue in the wound healing process due to its ability of rapid gelation [27]. However, fibrin application in 3D printing technology does not ensure the stability of the 3D structures due to its soft and fragile nature [35].
Another biomaterial in bioprinting technology is a Hyaluronic acid (HA) which is a part of ECM as linear polysaccharide [27]. Methacrylate group conjugation via free radical polymerization when exposed to UV light, is responsible for the HA found in the gels [27]. Employment of reconstituted basement members from mouse tumors like MatrigelTM and Cultrex® for the production of 3D printed tissues are also attractive biomaterials for the bioprinting process [32,36]. The inclusion of the basement membrane in a bioprinting polymer solution is attained by the maintenance of architecture and cell function improvement [32,37].
Synthetic and semi synthetic materials
Synthetic and semisynthetic materials have been derived to overcome the drawbacks that come forth by biological material. These synthetic materials are better alternative to biological materials. Synthetic material PCL, PEG, PLGA, PLA, and PGA are mostly used [38]. PEG is mostly used in tissue engineering applications. Hydrogels based on PEG are produced by crosslinking of chemical or UV light of functional polymers, allowing encapsulation of the cell with high viability. PEG hydrogels can be prepared by the incorporation of the ligand from oligopeptides to restore biochemical signaling e.g. Peptides of arginine-glycine-aspartic acid make integrin mediated cell adhesion and thus promote migration. Synthetic materials do not contain native ECM and are not biologically relevant as compared to natural materials [39]. Peptide hydrogels are used to create an environment for cell culture. Peptide sequences are used to make the peptide scaffold that enables the peptide scaffold to self-assemble under particular conditions. The natural microenvironment can be created by the use of cells that are able to generate natural ECM [40]. Bioinks can be used to fulfill the biological and mechanical aspects of target tissues. So the selection of bioinks requires evaluation of the physiology of organ. For example, the organs which bear load, the graft should be able to provide strength for bone tissue culturing. PCL is a good candidate in orthopedic applications. Mechanical and biological support of the 3D bioscaffold can be enhanced by the PCL matrix with hydroxyapatite particle. Synthetic and natural material used in biofabrication are given in the (Table 2) [41].
Types of 3D bioprinting
Depending upon the composition, structure, and printing quality of the bioink, there are two main forms, cellular and acellular bioprinting [26,42]. A cellular bioprinting is mainly categorized into direct implementation and post seeding. The non-living implant device or artificial graft substitute is required in a-cellular implant whereas cellular implant differs from a-cellular bioprinting in the employment of an additional cell depositing step following acellular bioprinting [42]. Certain factors such as surface resolution, the cellular capability of development and survival and biological material required for the printing purpose are essential for the selection of these representative techniques in 3-D bioprinting [23].
Biological material-based bioprinting
The utilization of living cells in the bio fabrication process with rapid prototyping is referred to cellular bioprinting. Here, cellular bioprinting is further divided into three categories; Droplet-based, extrusion-based and laser-based bioprinting, depending upon the printing modality of these techniques [42-44]. Droplet-based bioprinting essentially utilizes the bioink having encapsulated living cells with non-living materials for achieving the respective organ configuration by the utilization of several energy sources including thermal, electric, acoustic and pneumatic, etc [42]. This approach is derived from a conventional 2D inkjet printing technique with commercially available desktop ink-based printers [44-46]. Due to its clarity, dexterity, proficiency and high-throughput capability, it has wider applications in the pharmaceutical field [47].
Extrusion-based bioprinting is the commonly used and cost effective 3-D printing technique [44]. This strategy employs pressure-assisted bioprinting (PAB) for the development and construction of 3D tissue/organ analogues [5,48]. This technique utilizes solutions, pastes or dispersions as their biomaterials [5]. The diving forces used in this technique are pneumatic, mechanical [32] or electromagnetic [47], which are applied to deposit cell solution on a building media/substrate [32]. In contrast to inkjet bioprinting, extrusion-based printing has the ability to print continuous cylindrical lines instead of small discontinuous bioink droplets [25].
Laser based bioprinting essentially utilizes the laser beam as its energy source for the deposition of cell-laden bioink in a reservoir for the production of high-precision molded patterns [47]. Beam scanning or image projection modeling techniques are used for this purpose [47]. Unlike the inkjet and extrusion-based techniques, this approach does not make use of the nozzle thus, making it a more effective technique for the deposition of bioink with high cell densities and high viscosity [32].
Inkjet-based bioprinting
The inkjet printers (also termed as drop-on-demand or drop-by-drop bioprinters) are widely used printing systems for the biofabrication of tissues and organs [32]. These printers also have a broad range of biological applications. As, this approach is driven from 2D inkjet-based printers so, the bioprinting system is mostly similar in manufacturing to the 2D ink-based printers [25,44] except the cartridge uses biological material instead of ink and electronically controlled elevator stage is employed for the z-axis control instead of paper. Currently, the printing and management of biological materials with the agility, accuracy and high resolution is mainly accomplished with inkjet bioprinting [44]. The commonly used forces for liquid drops ejection from the nozzle onto a substrate are thermal [5,26,32,49-51] or acoustic [26,32,44,52].
This technique utilizes the hydrogel pre-polymer solution [25], containing biomaterials, growth factors (GFs) and encapsulated cells for its deposition on specified locations on a substrate [27,47]. The cartridge of inkjet bioprinters is fed with the bioink solution and then this solution further proceeds or dropout as droplets from the ink chamber [47]. There are two types of printing heads: thermal and piezoelectric [25], employed for the generation of droplets [5].
In thermal inkjet printers, heat is generated electrically [44] and this heat ranges from 200-300°C [26,44], is employed to generate the pressure pulses for the ejection of picoliters volume of droplets from bioprinter nozzle [32,44]. This temperature range is reported to have no apparent damaging effect on the biological molecules including DNA and living cells [53,54]. The generated heat mainly converts the bioink to vapor bubbles which releases from the printer orifice due to the pressure exerted for the bubble expansion and removal of expanded bubbles from the print head [55]. Due to its accessibility, rapid printing and cost effectiveness [44], thermal inkjet printers have a wide range of applications [5]. However, the efficiency of thermal inkjet printers gets lowered due to the blockage of the nozzle, the aberrant flow of droplets, uneven size of droplets and cellular material facing thermal stress [44]. However, the second type of head printer called the piezoelectric bioprinter (acoustic) is responsible for producing acoustic waves in the print head [32]. Additionally, piezoelectric crystal actuator generates pressure pulse by the application of voltage which modifies the shape of piezoelectric material and thus the pressure generated forces the droplets out of the orifice [44,47]. In contrast to the thermal inkjet printers, as there are no limiting factors such as the presence of volatile compounds and coagulation, involved so, it offers a greater range of bioinks. Moreover, the size and the shape of droplet and its ejection rate is affected by various factors including applied voltage, pulse duration and amplitude. Various ultrasound parameters and acoustic radiations are involved in the maintenance of drop size, droplet discharge rate and droplet discharge regularity. Furthermore, the utilization of acoustic frequencies in these printers is potentially damaging to the cell membranes, causing cell lysis [30] [47]. Moreover, complex tissue and organ construction is accomplished by the organization of various cell types and other tissue components by utilizing variants of inkjet techniques such as multi-jets [47,56].
Droplets can also be produced by the application of constant pneumatic pressure using the pneumatic pressure-assisted technique. This technique uses a set of electromechanical micro-valves with droplet ejection through the opening of the micro-valve after the application of constant pneumatic pressure [47]. Various types of liquid biomaterials having the viscosities up to 200 Pas are usually employed. Moreover, by regulating the pressure to a fluidic pathway, closing and opening duration (up to 200μs) of a valve control the volume of the droplets [27,47].
Another type of inkjet printing involves the electrohydrodynamic jetting (electrospraying or electrosppining) which is concerned with the application of electric potential difference between a positively charged needle and a grounded electrode for the production of repulsive coulombic force. During the course of transmission of charged medium from the needle to the high-intensity electric field results in the ejection of the droplet of size ranging from micrometer to nanometer. In relation to inkjet bioprinting, electrodynamic jetting is efficient in processing the concentrated suspensions from few a hundred micrometers size needles which can easily generate few micrometers and smaller sized droplet deposits [47]. Whereas, the inkjet bioprinting is associated with the production of a droplet with the diameter approximately two times the size of the jetting needle diameter.
Laser-assisted droplet bioprinting is another technique (also referred as laser-induced forward transfer or LIFT), which includes a pulsed laser source, a donor layer (having two layers; a laser- energy absorbing layer of gold or titanium and a bioink layer) and a receiving substrate. The mechanism involved in the ejection of droplets onto the substrate is the collision of a focused laser on the absorbing layer which consequently produces a high-pressure bubble that ultimately ejects the droplet onto the substrate. Additionally, the viscosity and thickness of the bioink layer determine volume of the droplet from 10 to 7000 pL. Due to its nozzle- free droplet model, this technique is efficient in depositing bioink with high cell densities (up to 108 cells/mL) and high viscosity (1-300 mPas) [47].
Extrusion-based bioprinting
Extrusion bioprinting is considered to be a widespread and cost-effective technique which is extensively applied in tissue biofabrication [32]. This approach comprises a dispensing (ejector or multiple ejectors) system and an automated three dimensional (x-y-z) robotic stage adjusted by the stage controller [32]. In extrusion printing, an air-force pump or a mechanical screw plunger is the driving force that is employed to expel bioinks. A constant force makes the release of continuous cylindrical lines from extrusion printer instead of droplets of bioink as in the case of extrusion printing [25]. There are mainly three types of dispensing systems: pneumatic, mechanical (piston or screw) and solenoid-based microextrusion [47].
The pneumatic-based approach is associated with the application of pressurized air to expel filaments through a valve-free or a value-based configuration. Due to certain characteristics such as controlled pressure, pulse frequency and the valve –based configuration is a more accurate as compared to valve-free configuration [47]. In contrast to the pneumatic-based system, mechanical microextrusion is more direct and simpler method of controlling bioink printing [47]. The low viscosity bioinks are usually dispensed by employing the piston system consisting of syringes and needles. However, the screw system is more efficient in the application of a larger pressure on the high viscosity bioinks [47,57]. Moreover, high viscosity bioinks including synthetic polymers are capable of generating the exact 3-D constructs by aiding it in structural maintenance, whereas during cell encapsulation lower-viscosity biomaterials, including hydrogels aid in the provision of a suitable environment that helps to maintain viability and function of cell [27].
Dispensing techniques are also associated with the printing of high density cells. Micro-sized nozzle facilitates the direct printing of cell-laden hydrogels or cell spheroids in syringes at the required position [27,58,59]. In addition, researchers mostly aim at utilizing thermally-cross linked materials and/or materials with shear-thinning properties in microextrusion bioprinting. Materials holding sheer thinning properties are extensively utilized for microextrusion applications [44]. It is observed that biomaterial exhibiting non-newtonian behavior shows an inverse relation between viscosity and sheer rate. The biomaterial which flows through the nozzle due to increased shear rates at the nozzle during biofabrication results in decreased sheer rates during deposition leading to the sharp rise in viscosity. An accurate fabrication of complex structures, designed using CAD software is achieved through high resolution of microextrusion systems. This also allows the designing of multiple cell types [44].
Extrusion based bioprinting have various strengths such as this technique permits the uniform distribution of cells due to high cell densities [27], it is also a time saving technique [27,60], can be easily operated, offer a wide range of bioinks, including cell aggregates, cell-laden hydrogels, synthetic polymer fibers, microcarriers, decellularized matrices [47]. This technique also permits up to 95% cell viability under the influence of various factors such as dispensing pressure, the nozzle size and cross-linking properties of hydrogels [61,62]. In contrast, there are certain weaknesses of extrusion-based bioprinting, including high shear pressure which bound the printing conditions of the cell-laden hydrogel [27], selection of material is restricted among others [47], low resolution as compared to other techniques [27], relatively low cell viability, 40-86% cell survival rates which inversely relates to the extrusion pressure and the nozzle gauge [44]. As the decreased cell viability may result in loss of resolution and printing speed so, this problem can be overcome by the usage of low pressures and large nozzle sizes [44]. Microextrusion bioprinters have been employed for the biofabrication of various types of tissues, such as aortic valves [44,63], branched vascular trees [64], in vitro pharmokinetics [65] and tumor models [44].
Stereolithography-based bioprinting
Stereolithography bioprinting technique appeared first in the 1980s. It is considered to be the oldest bioprinting approach which facilitates the generation of 3D complex structures with very high resolution and precision comparatively [27]. In spite of the use of solid form and nozzle system, a formulation having photosensitive liquid polymer is subjected to illumination to form a solid structure [5]. As, the polymeric material used in the technique is light sensitive so, the digital micromirror technique is exploited for the regulation of light intensity which is used for polymerization [7]. SCB is the appropriate technique for printing live cells as long as the photocurable polymer solution or a pre-polymer is utilized, which is subjected to a direct UV or laser light [7].
In this technique, a single beam laser is used for the polymerizerization or crosslinking of the photopolymer resin [66]. Spatially controlled irradiation of light or laser is employed in this technique (vat photopolymerization) by layering bioink through selective photopolymerization for the solidification of the geometric 2D patterns [47]. The stereolithography-based bioprinting technique generates 3D structure by continuously layering on 2D patterned layers whereas, the photo-polymerization of 2D patterned layers is considered to be the most crucial step in SLA-based bioprinting [47]. The induction of photo-polymerization in SLA-based bioprinting is mainly controlled by certain, factors including light intensity, irradiation time and the photo initiator concentration, and this induction is attained by either single-photon or two-photon absorptions [43,47]. Beaming-scanning and mask-image-projections are the two further categories of conventional SLA-based bioprinting techniques [47,67].
In the beam-scanning method, drawing and solidification of 2D patterns are achieved through the utilization of the selective scanning of a focused laser beam [27]. Factors affecting the resolution of the process include wavelength, power, exposure time and velocity, laser spot size, the occurrence of absorption or scattering of the laser beam, and the photo-initiator adopted or any UV absorbers [47,68,69].
The mask-image-projection process employs a digital light processing technique (DLP) for the generation of a defined mask image [58,70,71]. The DLP system utilizes an image generation device such as the digital micro mirror device, which is efficient in the solidification of one entire 2D layer by a single projection of the pattern image [27]. This consequently results in the generation of 2D pattern image within a short time duration [27]. Therefore, in comparison to the beam scanning process, mask-image-projection printing is rapid technique.
The SLA-based bioprinting commonly utilizes several types of photocurable bioinks including methacrylate/acrylate natural biomaterials (gelatin, hyaluronic acid, dextran and others, polyethylene glycol acrylate/methacrylate and its derivatives, and methacrylate/ acrylate capped among other synthetic polymers [42]. The strengths of SLA-based bioprinting techniques include high resolution complex patterned structures, printing with high speed and in lesser time duration, construction of complex structures without support material [47]. Moreover, μSLA systems are efficient in the generation of low to 50μm features with the smallest less than 5μm features [47,71]. However, besides the potential benefits of the SLA-based bioprinting technique, there are various shortcomings which include, free radical formation during photopolymerization causing potential damage to the cell membrane, proteins and nucleic acids, the less accessibility of photocurable materials and expensive equipment [47].
Synthetic material-based bioprinting
In contrast to cellular bioprinting, acellular 3D bioprinting has a wide range of options for method and material selection [47]. The acellular bioinks can be easily employed in the aforenamed cellular bioprinting techniques for the tissue engineered scaffolds fabrication. Artificial 3D bioprinting of tissues/organs can be accomplished by an additional cell seeding technique [47]. A universal cell seeding process or a bioreactor can be employed in the post-seeding process [47]. Moreover, the acellular 3D printing technique facilitates the transplantation of acellular 3D printed tissues for the functional replacement to the bruised patients or the provision of structural support during the healing process [47]. The acellular 3D bioprinting technique is further classified into extrusion-based bioprinting and laser-based bioprinting.
Extrusion-based bioprinting
Extrusion-based bioprinting technique differs from the aforementioned extrusion cellular bioprinting in the exploitation of volatile organic solvents for the formation of 3-D structures [47]. This involves the conversion of the highly viscous solution to solid 3D constructs by dissolving polymers from volatile organic solvents [47]. Here in, the cells are completely separated from the organic solvents which are then cultured on the surface of the scaffold for tissue/organ regeneration [47].
Fused deposition modeling or fused filament fabrication is a thermo-based tissue engineering technique for scaffold fabrication [66]. In this technique, filaments are heated up to their melting points which are the main source materials for the construction of 3D structures [70]. The extrusion nozzle is employed for the deposition of heated filaments [70]. Nozzles are involved in providing heat to melt the filaments in order to eject it onto the substrate for the biofabrication of 3D tissues and organs [70]. The melting temperature of the building material defines the temperature of the process which is generally responsible for cell death or for retardation of activity of bioactive molecules [66]. The dimensions (x, y and z) of the 3D structure are defined by computer aided programs that regulate the nozzle and substrate as well [70]. This technique is mainly derived from the extrusion or injection molding method which differs from FDM in the use of molds [70]. The components of the printer include heating blocks along with temperature regulators, an extrusion block and motors [47,58]. Two types of extrusion forces may be applied to the printing material, either pneumatic or mechanical [47]. Pati et al., [72], reported to exploiting a 3D printed scaffold made from a composite of polycaprolactone (PCL), poly (lactic-co-glycolic acid) (PLGA), and β-tricalcium phosphate and mineralized ECM to construct bone graft substitutes. Lee at al., [73], utilized melt-plotted/ in situ plasma-treated PCL scaffolds which are layered by chitosan of varying molecular weights. Hong et al., [74], reported to utilize 3D PCL/PLGA scaffolds for the solid freeform fabrication.
FDM technique provides various advantages in tissue engineering applications which includes easy operation, high printing speed, a wide range of synthetic biomaterials, mechanical properties best suited for hard tissue regeneration, and no requirement of solvent submersion [47]. In addition, various synthetic biomaterials have shown efficient thermo-plastic performance and bio compatibility including poly (caprolactone) (PCL), poly (lactic acid) (PLA), polyurethane and their derivatives [58].
As, the technique is best suited for those materials which can be melted and then re-shaped or thermally cross-linked, so, low-temperature thermoplastic is the preferred biomaterial for FDM whose function is maintained by adding various biologicals through mild processes [47]. However, the selection of low-temperature thermoplastic has confined the range of materials for biofabrication [47]. Another factor that limits the application of FDM is its high temperature requirement which makes the environment unfavorable for cells to print different structures and additional seeding of cells on constructs for biofabrication [47].
There are various desktop 3D printers available, which varies according to their cost. Several inexpensive 3D printers include Maker Bot, Ultimaker, Flashforge and Prusa [55]. However, these printers have limited applications due to the production of lower resolution constructs and a variety of materials being employed [55]. In addition, there are various expensive FDM printers such as Stratasys 3D printers that have relatively higher resolution of products produced and have the capability to exploit a wide variety of materials [55].
Laser-based bioprinting
Stereolithography technique is also associated with the fabrication of synthetic scaffolds [47]. As the acellular bioprinting has the least concerns related to cell rupture during the printing process so, there is the availability of more photocuring resins and crosslinking conditions [47]. Another laser-based printing technique includes selective laser sintering [47] which is extensively used in scaffold fabrication [66]. It is known to utilize a high- power laser, such as carbon dioxide laser, for polymer powder sintering for the scaffold formation. The scaffold is constructed in layer-layer fashion by polymer powder fusion into large parts [66]. SLA is the technique that does not require any support [66] as the print head and printing object has no connection with each other [55]. The technique utilizes two energy sources, a bed heater and a high-power laser [47]. The method began with the preheat treatment of particles at a temperature which lies between their melting transition and temperature require to recrystallize during the cooling cycle [47]. This technique relies on various types of materials including ceramics, metals, and composites [47].
There are certain factors that control and affect this technique such as the size and shape of particles, free packing density, energy source and thermodynamic variations of materials [47]. The resolution range of 20 to 100μm is usually attained and manipulated by obtaining a careful balance between the achievement of fine resolution and allowance for adequate powder dispensability [47]. 3D constructs are physically supported by the unsintered powders that are removed or reused after bioprinting [47]. Various characteristics that should be managed with great care in order to eliminate polymer deterioration due to overheating include power, beam size, scanning speed and spacing [47].
Selective laser sintering holds various advantages in the tissue/organ biofabrication process, such as easy accessibility of biomaterials including metals and ceramics employed for the fabrication of hard bone replacements or structural-supporting materials [47]. In addition, the materials which are more widely available for this technique are the powders [30] [47]. However, various limitations lessen the application of SLS due to its high cost, complex and laborious method and production of low resolution tough and hard chemical constructs [47]. However, various physical and chemical factors such as material oxidation, thermal deterioration, crystallinity change and material contraction influenced by the heating process also present problems to this technique [47]. Selective heat sintering (SHS) is another related technique associated with the exploitation of thermal print head instead of the utilization of laser for the formation of patterned, layered structures by fusing the surface of powdered thermoplastic materials [47].
Biomedical applications of 3D bioprinting
3D bioprinting has a wide range of applications in the biomedical field and has become an attractive technique in tissue engineering due to its various characteristics including geometry control and amount of biomaterial utilized [26]. Development of various organs including blood vessels [25,75], bone [5,76], cartilage [27,77], heart [5,78], kidneys [5,79], skin [5,26,80], neurons [25] and other tissues. In this section, some of the recent applications of bioprinting in the construction of various tissue types and in the new drug discovery are discussed.
Bioprinting of heart and vessels
As cardiovascular diseases (CVD) are the principle cause of worldwide death of humans so, its therapy and cure are critical for the survival of patients suffering from CVD [27]. CVD accounts for various associated diseases, including coronary artery diseases, for example, angina and myocardial infarction [27]. Now, here is the need for proper transport of nutrition, proper oxygen supply and functional blood vessels [5]. Therefore, in vitro organ construction with rich blood and oxygen supply is the attractive therapy of CVD [5]. Moreover, the challenge to this technique is the difficulty in developing vasculature specific to different tissues, however, this problem can be prevented with the help of novel bioprinting techniques [5,25].
3D bioprinting technique resolved the problem of vasculature fabrication using hydrogels or other bioinks. Dolati et al., [81], reported employing a coaxial nozzle system for the printing of more than a meter long vascular conduits. Various authors utilized this technique for the fabrication of conduits of diameters ranging up to submillimeter range but did not up to capillary diameters. Bertassoni et al., [82], utilized GelMA for the successful fabrication of vascular networks. GelMA enhanced metabolic transportation, cellular viability and the formation of endothelial monolayers. Kolesky et al., [83], printed different channels of diameter as small as 45μm with a bioink named Pluronic F127. This bioink in association with HUVECs further endothelialized the printed channels. Kolesky et al., [83], also utilized thermally reversible gelation for the construction of different vessels and complex tissues.
Extensive studies have been performed by researchers for the fabrication of aortic valve structure hydrogels [49,63,84]. As 50-90% of the cells lead to die off while injected through an extrusion and hostile environment was another principle cause of cell death [27]. So, this technique offered more than 90% of cell viability in the fabrication of cell laden, valve shaped structures [5].
Bioprinting of bone and cartilage
Due to the simplicity of composition (mainly of inorganic salts) of tough and stiff tissues, bone and cartilage are considered as the most established technique [5]. For bone and cartilage regeneration, there are various biomaterials available including gas foaming [85], freeze drying [86,87], and salt leaching [88,89]. In contrast to other technologies, 3D bioprinting is considered to be the most promising technique in precisely managing the structural and mechanical properties of artificial scaffolds [5]. Wang et al. [68], reported to exploiting poly (propylene fumarate) to print porous scaffolds and demonstrated it as an appropriate scaffold for bone tissue engineering applications. Castillho et al., [90], printed a cement powder system with the biomaterial containing HA and TCP which is considered to be the perfect composition for the human bone replacement to fix large defects. Pati et al., [72], utilized the human nasal inferior turbinate tissue-derived mesenchymal stomal cells which increase the osteogenic ability of PCL/PLGA/β-TCP scaffolds for the placement of bone-like ECM. Investigations under both invitro and invivo after culturing for a certain time period showed enhanced osteoinductive and osteoconductive properties [25].
Bioprinting technology has also stepped into the construction of cartilage tissue engineering scaffolds by the exploitation of various biomaterials [5]. These bioinks includes a wide variety of materials ranging from ceramics to nanomaterials [5]. Markstedtet et al, [77], reported to exploiting a printable bioink with a composition containing alginate, nanofibrillated cellulose and human chondrocytes as living soft tissue. Asfter culturing for 7 days, this printable bioink showed 86% cell viability with excellent shear-thinning properties [5,77]. Proper cell sources, proper hydrogels and growth factors (GFs) are the pre-requisites for cartilage printing [27,91,92]. The cell sources which are mostly employed in cartilage regeneration include mesenchymal stem cell (MSC), adipose derived stromal stem cell (ASC), and chondrocyte derived from OC and auricular cartilage [27,91,92]. Hydrogels used for cartilage reconstruction mainly include collagen (type I and II), gelatin, hyaluronic acid, and alginate hydrogel [27,91,92]. However, transforming growth factor- β1 (TGF- β1) and insulin like growth factor-1 are the GFs that provide a suitable environment for printing cartilage and for chondrogenesis [27,93-95]. Herein, the 3D bioprinting technique proved itself to be an ideal method for printing structures like bone and cartilage.
Nerve tissues
3D bioprinting has paved its way towards another application named as neuronal tissues. The previously discussed vasculature which is widely diffused throughout the whole body is seen in the nervous system as well [47]. It has an essential role in controlling all the biological processes via neuronal connections between the brain and all parts of the body [47]. As, the injuries that usually occur in the central nervous system (CNS) cannot be healed due to the absence of schwann cells (SCs), therefore, tissue engineering techniques and scaffolds for tissue/organ regeneration are the most convenient and appropriate methods for CNS fabrication [47]. Lozano et al., [96], created an artificial multilayered structure of cortical tissue with natural hydrogel composed of a natural RGD peptide-modified gellan gum [32]. Extrusion based 3D printers were employed for the construction of multilayers of neural cells which formed after 5 days of 3D culture [32]. Another study conducted by Lorber et al., [97], showed the construction of retinal ganglion cell (RGC) neurons and retinal glial cells of the rat with the help of piezoelectric droplet bioprinter. Pateman et al., [98], also proved the idea of printing cells of the nervous system by printing PEG-based nerve guidance conduits with the help of micro-stereolithographic technique for nerve repair studies [25]. Development of nerve tissues is actually the exploitation of ability of 3D bioprinting to print biomaterials in highly controlled dimensions [26,99].
Developments in bioprinting techniques
Development in bioprinting technique involves continuous liquid interference production (CLIP) aided by oxygen inhibit the dead zone that prevents the attachment of resin to the UV window. CLIP uses a thin amorphous Teflon film for permeabilization of oxygen, an oxygen containing zone is created between solid part and the liquid precursor. The rate of formation of tissue in the layer by layer fashion is determined by initiation efficiency and resin reactivity. CLIP has made 3D printing to complete in minutes instead of hours as in the case with traditional SLA. Structures produced by CLIP contain resolution power below100 µm. In CLIP, however, the reactivity of monomers is the crucial factor because it affects the oxygen diffusion and thus permeability of the window is affected by it [100]. The 3D power printing technique is based on the principles of SLS. This technique uses citric acid, water or phosphoric acid as the binder solution to bind loosely powdered material in a defined geometry. Bio materials that are used as binder integrin are hydroxyapatite, calcium phosphate, gelatin, dextrin and starch. This technique avoids the damage of incorporated bioactive components and thus is more efficient for drug delivery and tissue engineering. However, this approach is unable to remove unbound powder from hollow spaces. Also, the use of liquid binder reduces the mechanical strength [101]. The two photon polymerization printing or nano-stereolithography uses simultaneous two photon absorption to photocure the liquid polymer. In SLA single photon polymerization is used, unlike these two photon polymerization allow the transition of electrons over excited energy levels. Polymerization is initiated by the combined energy of two photons with the low wavelength absorbed by a photoinitiator. Polymerization is triggered when nonlinear excitation occurs at the focal point, but other regions remain safe from laser energy. In this way, this technique is able to print 3D structures with high resolution. This approach can achieve resolution with up to 100nm. Its high resolution is used to check the environment for 3D printing for cell proliferation and adhesion. This is a highly efficient technique, but its materials and process cost has confined it to a small scale [42].
Limitations for bioprinting
3D bioprinting has wide applications ranging from cellular behavior to tissue pharmacodynamics. This technique has high reproducibility, high precision, but the problem is layer by layer assembly of tissues with bio-glue. Suitable bioink with biological compatibility is the barrier to achieve proper biological function. For soft tissues, hydrogels are used and for hard tissues, ceramics are used. For tthe sustainable development of 3D technology, it is essential to regulate laws and regulations. Progress is required to technique has high reproducibility, high precision but the problem is layer by layer assembly of tissues with bio- print micro organs tissue that must act efficiently in the absence of the structure of that specified organ e.g. in pancreas islet tissues in case of diabetic patients. With the progress of this technology we hope that in the future this technology may be able to develop additional biomimetic and tissue engineered organs, reestablishment and time and cost will also be decreased in the applied clinic [5]. This technology has limited application due to the lack of diversity of biomaterials, biomaterial printability, biodegradation, biocompatibility properties and suitable mechanical strength for culturing of tissue. Vascularization is important for any scaffold to be functional, and this is impossible with current 3D bioprinting technology. This problem is solved by the incorporation of sacrificial material during the formation of a scaffold. This material provides mechanical support to printing material by filling the void spaces. These materials are removed by post processing after the fabrication. Limitation induced by the design cause discontinuity of material due to the poor efficiency of CAD design into the machine [55]. Although 3D bioprinting significantly progressed over the years, its limitations leads to the development of 4D bioprinting technology [102].
Concluding remarks and future aspects
3D bioprinting is a technique revolving around different fields such as engineering, biological sciences, computer science and medicine which is considered as the potent technique for the construction of different tissues and organs of varying structural and functional complexity [47]. However, various challenges related to 3D bioprinting techniques such as low resolution, slow printing, less availability of relevant biomaterials suitable for printing specific tissues/organs [47]. In addition, the development and simulation of microenvironments from molecular to macroscopic scales for the generation of tissues/organs is a major challenge in the bioprinting technique [32]. Despite the development and progress in this field of tissue engineering such as microfluidic systems [103,104], biopatterning [105], and layer by layer assembly [106], biomanufacturing of micro-tissue constructs within scaffolds or without scaffolds, there are great challenges lying ahead such as vascularization which causes hypoxia, apoptosis and immediate cell death. So, this limitation needs to be dealt with immediately by developing an effective solution for vascularization [32]. However, various efforts have been made such as the fabrication of porous scaffolds which although provide enough space for vascularization but the diffusion of cells and other materials into the pores makes vascularization challenge difficult to overcome [32]. Once, the aforementioned challenges are met, the bioprinting technology will enable more improvement in rapid clinical solutions and advancements in in vitro screening, diagnostic applications [32], cancer biology, tissue engineering and regenerative medicine [107-109].
3D bioprinting is the advanced tissue engineering technique providing an ideal solution to the worldwide pressing problem of organ shortage. It is rapidly expanding biofabrication technique with various applications including patient-specific implants, engineering scaffolds for tissue regeneration, personalized drug delivery [55]. Despite the rapid improvement in the 3D modeling software, accurate geometry, speed and regulation of 3D printers and development in the mechanics of printers [55], the 3D bioprinting technology is still taking baby steps, but have an excellent capability to excel and progress in tissue engineering applications.
- Gopinathan J, Noh I (2018) Recent trends in bioinks for 3D printing. Biomater Res 22: 11-15. Link: https://bit.ly/2L0W5Tp
- Gu BK, Choi DJ, Park SJ, Kim MS, Kang CM, et al. (2016) 3-Dimensional Bioprinting for Tissue Engineering Applications. Biomater Res 20: 12. Link: https://bit.ly/2KYA1ZY
- Sindhuja SNR, Eswaramoorthy D, Ramakrishna S (2019) Recent advances in three‐dimensional bioprinting of stem cells. Journal of Tissue Engineering and Regenerative Medicine 13: 908-924. Link: https://bit.ly/2FRmbV1
- Gao G, Cui X (2016) Three-dimensional bioprinting in tissue engineering and regenerative medicine. Biotechnol Lett 38: 203-211. Link: https://bit.ly/2JbAbuw
- Li J, Chen M, Fan X, Zhou H (2016) Recent advances in bioprinting techniques : approaches , applications and future prospects. J Transl Med 14: 271. Link: https://bit.ly/2FON6k8
- Wu GH, Hsu SH (2015) Review: Polymeric-based 3D printing for tissue engineering. J Med Biol Eng 35: 285-292. Link: https://bit.ly/2XmLTeN
- Ji S, Guvendiren M (2017) Recent Advances in Bioink Design for 3D Bioprinting of Tissues and Organs. Front Bioeng Biotechnol 5: 23. Link: https://bit.ly/2YxY0C2
- Hong N, Yang G, Lee J, Kim G (2017) 3D bioprinting and its in vivo applications. J Biomed Mater Res B Appl Biomater 106: 444-459. Link: https://bit.ly/2RQKYxc
- Li YC, Zhang YS, Akpek A, Shin SR, Khademhosseini A (2017) 4D bioprinting: The next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication 9: 012001. Link: https://bit.ly/2xy2k8s
- Huh D, Torisawa YS, Hamilton GA, Kim HJ, Ingber DE (2012) Microengineered physiological biomimicry: Organs-on-Chips. Lab Chip 12: 2156-2164. Link: https://bit.ly/2Jh5TFy
- Reed EJ, Klumb L, Koobatian M, Viney C (2009) Biomimicry as a route to new materials: What kinds of lessons are useful? Philos Trans R Soc. A Math Phys Eng Sci 367: 1571–1585. Link: https://bit.ly/2LD228A
- Ingber DE (2007) Tissue Engineering and Developmental Biology: Going Biomimetic. Tissue Eng 12: 3265–3283. Link: https://bit.ly/2JmPQ9h
- Article R (2018) 3D-Bioprinting: A stepping stone towards enhanced medical approaches. Advancements in Life Sciences 5: 143–153. Link: https://bit.ly/2LCYZ0r
- Marga F, NeaguA, Kosztin I, Forgacs G (2007) Developmental biology and tissue engineering. Birth Defects Res C Embryo Today 81: 320–328. Link: https://bit.ly/2FRbqCa
- Steer DL, Nigam SK (2004) Developmental approaches to kidney tissue engineering. Am J Physiol Physiol 286: F1–F7. Link: https://bit.ly/2J9zguJ
- Derby B (2012) Printing and prototyping of tissues and scaffolds. Science 338: 921–926. Link: https://bit.ly/2FOQDPh
- Kasza KE, Rowat AC, Liu J, Angelini TE, Brangwynne CP, et al. (2007) The cell as a material. Curr Opin Cell Biol 19: 101–107. Link: https://bit.ly/2YFTLop
- Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, et al. (2009) Organ printing: tissue spheroids as building blocks. Biomaterials 30: 2164–2174. Link: https://bit.ly/2XKCjSi
- Kelm JM (2010) A novel concept for scaffold-free vessel tissue engineering: Self-assembly of microtissue building blocks. J Biotechnol 148: 46–55. Link: https://bit.ly/2RTrpo3
- Sonntag F, Schilling N, Mader K, Gruchow M, Klotzbach U, et al. (2010) Design and prototyping of a chip-based multi-micro-organoid culture system for substance testing, predictive to human (substance) exposure. J Biotechnol 148: 70–75. Link: https://bit.ly/309PedV
- Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Yuan Hsin H, et al (2010) Reconstituting organ-level lung functions on a chip. Science 328: 1662–1668. Link: https://bit.ly/2XLWsaD
- Murphy SV, Atala A (2014) 3D bioprinting of tissues and organs. Nat Biotechnol 32: 773–785. Link: https://bit.ly/2LCoZsH
- Murphy SV, Atala A (2014) 3D bioprinting of tissues and organs. Nat Biotechnol 32: 773–785. Link: https://bit.ly/2LCoZsH
- Jammalamadaka U, Tappa K (2018) Recent Advances in Biomaterials for 3D Printing and Tissue Engineering. J Funct Biomater 9: E22. Link: http://bit.ly/2Xr5PYS
- Mandrycky C, Wang Z, Kim K, Kim D (2017) 3D Bioprinting for Engineering Complex Tissues. Biotechnol Adv 34: 422–434. Link: https://bit.ly/2XOYkiN
- Hong N, Yang G, Leev, Kim G (2017) 3D bioprinting and its in vivo applications. 106: 444–459. Link: https://bit.ly/2RQKYxc
- Park JH, Jang J, Lee JS (2016) Three-Dimensional Printing of Tissue / Organ Analogues Containing Living Cells. Ann Biomed Eng 45: 180-194. Link: https://bit.ly/2JcpAzS
- Guillotin B, Guillemot F (2011) Cell patterning technologies for organotypic tissue fabrication. Trends Biotechnol 29: 183–190. Link: http://bit.ly/2xw9UAQ
- Gurkan UA, Tasoglu S, Kavaz D, Demirel MC, Demirci U (2012) Emerging Technologies for Assembly of Microscale Hydrogels. Adv Healthc Mater 149–158. Link: http://bit.ly/2YzrkIm
- Guvendiren M, Burdick JA (2013) Engineering synthetic hydrogel microenvironments to instruct stem cells. Curr Opin Biotechnol 24: 841–846. Link: http://bit.ly/2YxXPqr
- Nguyen MK, Alsberg E (2014) Bioactive factor delivery strategies from engineered polymer hydrogels for therapeutic medicine. Prog Polym Sci 39: 1236-1265. Link: http://bit.ly/2JkyPg4
- Arslan-yildiz A, El Assal R, Chen P, Guven S, Inci F, et al, (2016) Towards artificial tissue models : past , present , and future of 3D bioprinting. Biofabrication 8: 014103. Link: https://bit.ly/2Jmflrx
- Malda J (2013) 25th Anniversary Article : Engineering Hydrogels for Biofabrication. Advanced Materials 25: 5011–5028. Link: http://bit.ly/2NtB6dZ
- Fedorovich NE, Alblas J, Ph D, Wijn JRDE, Ph D (2007) Hydrogels as Extracellular Matrices for Skeletal Tissue Engineering: State-of-the-Art and Novel Application in Organ Printing. Tissue Eng 13: 1905-1925. Link: http://bit.ly/2RYxmjA
- NakamuraM, Iwanaga S, Henmi C, Arai K, Nishiyama Y (2010) Biomatrices and biomaterials for future developments of bioprinting and biofabrication. Biofabrication 2: 014110. Link: http://bit.ly/2XPTVfv
- Kleinman HK, Martin GR (2005) Matrigel : Basement membrane matrix with biological activity. Semin Cancer Biol 15: 378–386. Link: http://bit.ly/2FTxj3L
- Poldervaart MT (2014) Prolonged presence of VEGF promotes vascularization in 3D bioprinted scaffolds with de fi ned architecture. J Control Release 184: 58–66. Link: http://bit.ly/32cKBSu
- Watson BM, Tiffany N, Tatara AM, Shah SR, Scott DW, et al. (2015) Biodegradable, phosphate-containing, dual-gelling macromers for cellular delivery in bone tissue engineering. Biomaterials 67: 286–296. Link: http://bit.ly/2L0GNhO
- Sasai Y (2013) Cytosystems dynamics in self-organization of tissue architecture. Nature 493: 318–326. Link: http://bit.ly/2KZjuVg
- Bourgine PE, Scotti C, Pigeot S, Tchang LA, Todorov A, et al. (2014) Osteoinductivity of engineered cartilaginous templates devitalized by inducible apoptosis. Proc Natl Acad Sci 111: 17426–17431. Link: http://bit.ly/2YzydJR
- Cho D (2016) Towards artificial tissue models: past, present, and future of 3D bioprinting. Biofabrication 8: 014103. Link: http://bit.ly/2XJVhIJ
- Cui H, Nowicki M, Fisher JP, Zhang LG (2017) 3D Bioprinting for Organ Regeneration. Adv Healthc Mater 6. Link: https://bit.ly/2Nwna2W
- Billiet T, Vandenhaute M, SchelfhoutJ, Van Vlierberghe S, Dubruel P (2012) A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 33: 6020–6041. Link: https://bit.ly/2RUqC6k
- Murphy SV, Atala A (2014) 3D bioprinting of tissues and organs. Nat Biotechnol 32: 773–785. Link: https://bit.ly/2LCoZsH
- Nicodemus GD, Bryant SJ, PhD (2008) Cell Encapsulation in Biodegradable Hydrogels for Tissue Engineering Applications. Tissue Eng Part B Rev 14: 149-165. Link: https://bit.ly/32aTY4M
- Wüst S, Godla ME, Müller R, Hofmann S (2014) Tunable hydrogel composite with two-step processing in combination with innovative hardware upgrade for cell-based three-dimensional bioprinting. Acta Biomater 10: 630–640. Link: https://bit.ly/2FSeVrE
- Cui H, Nowicki M, Fisher JP, Zhang LG (2018) 3D Bioprinting for Organ Regeneration. Adv Healthc Mater 6: 1–54. Link: https://bit.ly/2Nwna2W
- Chia HN, Wu BM (2015) Recent advances in 3D printing of biomaterials. J Biol Eng 9: 4. Link: https://bit.ly/2YwdnuN
- Xiaofeng Cui MKL, BolandT, Darryl D, D’Lima (2013) Thermal Inkjet Printing in Tissue Engineering and Regenerative Medicine. Recent Pat Drug Deliv Formul 6: 149–155. Link: https://bit.ly/2L5xHjV
- Cui X, Dean D, Ruggeri ZM, Boland T (2010) Cell Damage Evaluation of Thermal Inkjet Printed Chinese Hamster Ovary Cells. Biotechnol Bioeng 106: 963–969. Link: https://bit.ly/307yCUj
- Cui X, Boland T (2009) Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials 30: 6221–6227. Link: https://bit.ly/2JohJ0P
- Okamoto T, Suzuki T, Yamamoto N (2000) Microarray fabrication with covalent attachment of DNA using Bubble Jet technology. Nat Biotechnol 18: 438-441. Link: https://bit.ly/325TpJC
- Xu T, Jin J, Gregory C, Hickman JJ, Boland T (2005) Inkjet printing of viable mammalian cells. Biomaterials 26: 93–99. Link: https://bit.ly/2JaHm6e
- Xu T, Gregory CA, Molnar P, Cui X, Jalota S, et al. (2006) Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials 27: 3580–3588. Link: https://bit.ly/3264omx
- Tappa K, Jammalamadaka U (2018) Novel Biomaterials Used in Medical 3D Printing Techniques. J Funct Biomater 9: E17. Link: https://bit.ly/2LEsNtp
- Xu T, Zhao W, Zhu J, Albanna MZ, Yoo JJ, et al. (2013) Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials 34: 130–139. Link: https://bit.ly/2KXmrWu
- Ozbolat IT, Hospodiuk M (2015) Current Advances and Future Perspectives in Extrusion-based Bioprinting. Biomaterials 76: 321-343 Link: https://bit.ly/2KWUQor
- Stansbury JW, Idacavage MJ (2015) 3D printing with polymers : Challenges among expanding options and opportunities. Dent Mater 32: 54–64. Link: https://bit.ly/2JcKb6Z
- Zhu W, Ma X, Gou M, Mei D, Zhang K, et al. (2016) 3D printing of functional biomaterials for tissue engineering. Curr Opin Biotechnol 40: 103–112. Link: https://bit.ly/2KYKuEF
- Do A, Khorsand B, Geary SM, Salem AK (2015) 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv Healthc Mater 4: 1742–1762. Link: https://bit.ly/2JciQBS
- Stewart RL (2004) Three-Dimensional BioAssembly Tool for Generating Viable Tissue-Engineered Constructs. Tissue Eng 10: 1566–1576. Link: https://bit.ly/2Nt1GEa
- Chang R, Nam JAE, Sun WEI (2008) Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication-based direct cell writing. Tissue Eng Part A 14: 41-48. Link: https://bit.ly/2Ja9Gpm
- Duan B, Hockaday LA, Kang KH, Butcher JT (2012) 3D Bioprinting of heterogeneous aortic valve conduits with alginate / gelatin hydrogels. J Biomed Mater Res A 101: 1255–1264. Link: https://bit.ly/2xqY1fo
- Norotte C, Marga FS, Niklason LE, Forgacs G (2009) Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 30: 5910–5917. Link: http://bit.ly/2YwXWmj
- Chang R, Nam J, Sun W (2008) Direct Cell Writing of 3D Microorgan for In Vitro Pharmacokinetic Model. Tissue Eng Part C Methods.14: 157-166. Link: http://bit.ly/2Yw1K7i
- WuGH, Hsu SH (2015) Review : Polymeric-Based 3D Printing for Tissue Engineering. J Med Biol Eng 35: 285–292. Link: http://bit.ly/2Jch2sI
- Zorlutuna P, Annabi N, Camci-Unal G, Nikkhah M, Cha JM, et al. (2018) Microfabricated Biomaterials for Engineering 3D Tissues. Adv Mater 24: 1782-1804. Link: http://bit.ly/2JaXi8M
- Zhu W, Wang M, Fu Y, Castro NJ, Fu SW, et al. (2015) Engineering a biomimetic three-dimensional nanostructured bone model for breast cancer bone metastasis study. Acta Biomater 14: 164–174. Link: http://bit.ly/2xx5xp3
- Miao S, Zhu W, Castro NJ, Nowicki M, Zhou X, et al. (2016) 4D printing smart biomedical scaffolds with novel soybean oil epoxidized acrylate. Sci Rep 6: 1–10. Link: http://bit.ly/2XqLH9w
- Gu BK, Choi DJ, Park SJ, Kim MS, Kang CM, et al. (2016) 3-dimensional bioprinting for tissue engineering applications. Biomater Res 20: 12. Link: http://bit.ly/2xtEbjw
- Skoog SA, Goering PL, Narayan RJ (2014) Stereolithography in tissue engineering. J Mater Sci Mater Med 25: 845-856. Link: http://bit.ly/2Joes1v
- Pati F, SongT, Rijal G, JangJ, Won S, et al. (2014) Ornamenting 3D printed scaffolds with cell-laid extracellular matrix for bone tissue regeneration. Biomaterials 37: 230-240. Link: http://bit.ly/2RSVJz3
- KyoungHo Lee GK, Jin GH, Jang CH (2013) Preparation and characterization of multi-layered poly(ε-caprolactone)/chitosan scaffolds fabricated with a combination of melt-plotting/in situ plasma treatment and a coating method for hard tissue regeneration. J Mater Chem B 1: 5831–5841. Link: https://rsc.li/2XKB859
- Hong JM, Kim BJ, Shim JH, Kang KS, Kim KJ , et al. (2012) Enhancement of bone regeneration through facile surface functionalization of solid freeform fabrication-based three-dimensional scaffolds using mussel adhesive proteins. Acta Biomater 8: 2578–2586. Link: http://bit.ly/2XJUEil
- Skardal A, Zhang J, Prestwich GD (2010) Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials 31: 6173–6181. Link: http://bit.ly/2NBd3dk
- Sawkins MJ, Mistry P, Brown BN, Shakesheff KM, Bonassar LJ, et al. (2015) Cell and protein compatible 3D bioprinting of mechanically strong constructs for bone repair. Biofabrication 7: 035004. Link: http://bit.ly/2Xo4rLA
- Markstedt K, Mantas A, Tournier I, Ha D, Gatenholm P (2015) 3D Bioprinting Human Chondrocytes with Nanocellulose − Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 16: 1489–1496. Link: http://bit.ly/2RS4jy3
- Beyersdorf F (2014) Three-dimensional bioprinting : new horizon for cardiac surgery. Eur J Cardiothorac Surg 46: 339–341. Link: http://bit.ly/30bxZJk
- Bernhard JC (2015) Personalized 3D printed model of kidney and tumor anatomy : a useful tool for patient education. World J Urol 34: 337-345. Link: http://bit.ly/2NIk0cq
- Skardal A, Mack D, Kapetanovic E, Atala A, Jackson JD, et al. (2012) Bioprinted Amniotic Fluid-Derived Stem Cells Accelerate Healing of Large Skin Wounds 1: 792–802. Link: http://bit.ly/2Xt8AOc
- Dolati F, Yu Y, Zhang Y, De Jesus AM, Sander EA, et al. (2014) In vitro evaluation of carbon-nanotube- reinforced bioprintable vascular conduits. 25: 145101. Link: http://bit.ly/2NvXUK9
- Bertassoni LE, Cecconi M, Manoharan V, Nikkhah M, Hjortnaes J, et al. (2014) Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip 14: 2202–2211. Link: http://bit.ly/2Jdsap6
- Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, et al. (2014) 3D Bioprinting of Vascularized , Heterogeneous Cell-Laden Tissue Constructs. Adv Mater 26: 3124–3130. Link: http://bit.ly/30g8AxZ
- Hockaday LA (2012) Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication 4: 035005. Link: http://bit.ly/2JrhZfk
- Wenchuan Chen HHKX, Zhou H, Tang M, Weir MD, Bao C (2012) Gas-Foaming Calcium Phosphate Cement Scaffold Encapsulating Human Umbilical Cord Stem Cells. Tissue Eng Part A 18: 816–827. Link: http://bit.ly/2xxjVNU
- Gerçek I, Tigli RS, Gümüşderelioglu M (2007) A novel scaffold based on formation and agglomeration of PCL microbeads by freeze-drying. J Biomed Mater Res A 86: 1012–1022. Link: http://bit.ly/2JozRYx
- Alizadeh M, Abbasi F, Khoshfetrat AB, Ghaleh H (2013) Microstructure and characteristic properties of gelatin / chitosan scaffold prepared by a combined freeze-drying / leaching method. Mater Sci Eng C Mater Biol Appl 33: 3958–3967. Link: http://bit.ly/2LBNy91
- Kim TG, Chung HJ, Park TG (2008) Macroporous and nanofibrous hyaluronic acid / collagen hybrid scaffold fabricated by concurrent electrospinning and deposition / leaching of salt particles. Acta Biomater 4: 1611-1619. Link: http://bit.ly/2YwjDTf
- Mehrabanian M, Nasr-esfahani M (2011) HA / nylon 6 , 6 porous scaffolds fabricated by salt-leaching / solvent casting technique : effect of nano-sized filler content on scaffold properties. Int J Nanomedicine 6: 1651–1659. Link: http://bit.ly/2XQlOUA
- Castilho M, Moseke C, Ewald A, Gbureck U (2014) Direct 3D powder printing of biphasic calcium phosphate scaffolds for substitution of complex bone defects. Biofabrication 6: 015006. Link: http://bit.ly/2XQMZPa
- Fedorovich NE, Schuurman W, Wijnberg HM, Prins HJ, van Weeren PR, et al. (2012) Biofabrication of Osteochondral Tissue Equivalents by Printing Topologically Defined. Tissue Eng Part C Methods 18: 33-44. Link: http://bit.ly/2RWFSPP
- Lee J, Hong JM, Jung JW, Shim J, Oh J, et al. (2014) 3D printing of composite tissue with complex shape applied to ear regeneration Biofabrication 6: 024103. Link: http://bit.ly/2Je29WP
- Madry H, Rico AR, Venkatesan JK (2013) Transforming Growth Factor Beta-Releasing Scaffolds for Cartilage Tissue Engineering. Tissue Eng Part B Rev 20: 106-125. Link: http://bit.ly/2YB0nnU
- Park H, Temenoff JS, Tabata Y, Caplan AI, Mikos AG (2007) Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials 28: 3217–3227. Link: http://bit.ly/2YtAV3z
- Spiller KL, Liu Y, Holloway JL, Maher SA, Cao Y, et al. (2012) A novel method for the direct fabrication of growth factor-loaded microspheres within porous nondegradable hydrogels : Controlled release for cartilage tissue engineering. J Control Release 57: 39–45. Link: http://bit.ly/32dSFlZ
- Lozano R, Stevens L, Thompson BC, Gilmore KJ, Gorkin R, et al. (2015) 3D printing of layered brain-like structures using peptide modi fi ed gellan gum substrates. Biomaterials 67: 264–273. Link: http://bit.ly/2RRqZyk
- Lorber B, HsiaoW, Hutchings IM, Martin KR (2014) Adult rat retinal ganglion cells and glia can be printed by piezoelectric inkjet printing. Biofabrication 6: 015001. Link: http://bit.ly/2FTMWIb
- Pateman CJ, Harding AJ, Glen A, Taylor CS, Christmas CR, et al. (2015) Nerve guides manufactured from photocurable polymers to aid peripheral nerve repair. Biomaterials 49: 77–89. Link: http://bit.ly/2NBLyAg
- Zhu W (2014) 3D nano / microfabrication techniques and nanobiomaterials for neural tissue regeneration. Nanomedicine (Lond) 9: 859–875. Link: http://bit.ly/2Nz0nUs
- Tumbleston JR (2015) Continuous liquid interface production of 3D objects (Supplementary Materials). Science 347: 1349–1352. Link: http://bit.ly/2Xsun8T
- Jose RR, Rodriguez MJ, Dixon TA, Omenetto F, Kaplan DL (2016) Evolution of Bioinks and Additive Manufacturing Technologies for 3D Bioprinting. ACS Biomater Sci Eng 2: 1662–1678. Link: http://bit.ly/2RUy8Oc
- Yang GH, Yeo M, Koo YW, Kim GH (2019) 4D Bioprinting: Technological Advances in Biofabrication. Macromol Biosci 19: e1800441: 1800441. Link: http://bit.ly/2Xr8UrU
- Nakao Y (2011) Bile canaliculi formation by aligning rat primary hepatocytes in a microfluidic device Bile canaliculi formation by aligning rat primary hepatocytes in a microfluidic device. Biomicrofluidics 5: 022212. Link: http://bit.ly/2Nz1inB
- Chang R, Emami K, Wu H, Sun W (2010) Biofabrication of a three-dimensional liver micro-organ as an in vitro drug metabolism model. Biofabrication 2: 045004. Link: http://bit.ly/2LEfCIQ
- Kuo WP, Demirci U (2014) Engineering Anisotropic Biomimetic Fibrocartilage Microenvironment by Bioprinting Mesenchymal Stem Cells in Nanoliter Gel Droplets. Mol Pharm 11: 2151–2159. Link: http://bit.ly/32bdC0v
- Snyder JE, Hamid Q, Wang C, Chang R, Emami Ket al. (2011) Bioprinting cell-laden matrigel for radioprotection study of liver by pro-drug conversion in a dual-tissue microfluidic chip. Biofabrication 3: 034112. Link: http://bit.ly/2RSAQUJ
- Rizvi I, Gurkan UA, Tasoglu S, Alagic N, Celli JP, et al. (2013) Flow induces epithelial-mesenchymal transition , cellular heterogeneity and biomarker modulation in 3D ovarian cancer nodules. Proc Natl Acad Sci USA 110: E1974-1983. Link: http://bit.ly/2JdY44V
- Gurkan UA, Anand T, Tas H, Elkan D, Akay A, et al. (2011) Controlled viable release of selectively captured label-free cells in microchannels. Lab Chip 11: 3979–3989. Link: http://bit.ly/2FQbIZO
- Gurkan UA, Tasoglu S, Akkaynak D, Avci O, Unluisler S, et al. (2012) Smart Interface Materials Integrated with Microfl uidics for On-Demand Local Capture and Release of Cells. Adv Healthc Mater 1: 661-668 Link: http://bit.ly/2XsxkGp
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
