Biomed Sci Eng
Peg-based temperature sensitive nanoparticle synthesis and their use in protein adsorption
Guldem Utkan*
Cite this as
Utkan G (2018) Peg-based temperature sensitive nanoparticle synthesis and their use in protein adsorption. Biomed Sci Eng 4(1): 021-024. DOI: 10.17352/abse.000010In this study, temperature sensitive polymeric nanoparticles were prepared by emulsifier free emulsion polymerization. N-isopropylacrylamide (NIPA) has been used as main monomer to give temperature sensitivity and poly(ethyleneglycol) ethyl ether methacrylate (PEG-EEM) has been added to recipes as comonomer to aid in particle formation. The effect of NIPA, PEG-EEM and crosslinker content on particle size has been investigated. Temperature sensitivity or lower critical solution temperature (LCST) of the nanoparticles has been followed by Zeta Sizer as change in particle size. In order to determine the protein adsorption capacity of nanoparticles, adsorption experiments have been investigated as a function of temperature and PEG-EEM amount. The results showed that the adsorption capacity of both NIPA and NIPA/PEG-EEM latexes was increased similarly with the increase in temperature. However, amount of BSA adsorbed on NIPA/PEG-EEM particles has been slightly higher than that of for NIPA alone. This study showed that synthesized NIPA/PEG-EEM nanoparticles would be a good candidate for the further studies on protein adsorption.
Abbreviations
NIPA: N-isopropylacrylamide; PEG-EEM: Poly (ethyleneglycol) ethyl ether methacrylate; LCST: Lower Critical Solution Temperature; PNIPA: Poly(N-Isopropylacrylamide); KPS: Potassium Persulphate; MBA: N, N’-methylene bisacrylamide; BSA: Bovine Serum Albumin
Introduction
Stimuli-responsive polymers that respond with dramatic property change to small changes in their environment. They can be classified according to the stimuli they respond to as: temperature, pH, ionic strength, light, electric and magnetic field sensitive. They are mostly used in the field of biomimetic actuators, immobilized biocatalysts, drug delivery, thermoresponsive surfaces, bioseparation and bioconjugates [1,2].
Important recent advances in poly(N-isopropylacrylamide) (PNIPA) based systems have focused on mechanistic understanding of phase separation, fine control of the structure-property relationship and novel biomedical applications. PNIPA is soluble below 32°C and precipitates above 32°C in water [3].
The adsorption of proteins on monodisperse latex particles has been widely studied in the field of biomedical applications, such as artificial tissues and organs, drug delivery systems, biosensors, solid phase immunoassays, immunomagnetic cell separations and immobilized enzymes and catalysts [1-4]. Various researchers have studied adsorption of proteins. Some of them concluded that hydrophobic interaction is the most important aspect of protein adsorption. [5-8]. Others claimed that hydrogen bonding is more important then hydrophobic interactions, in which they used the copolymerized particles with acrylate and acrylic acid (carboxylated) [9,10]. Polystyrene latex particles were found quite useful for promoting protein adsorption through hydrophobic interactions [4-6]. These particles had long been considered as colloidal polymer models for protein adsorption onto polymeric supports. Protein adsorption onto such latexes exhibited generally a rapid and irreversible process, hydrophobic and electrostatic interactions govern this process, hence the physicochemical properties of two participating components play a predominant role, as well as the external conditions (pH, ionic strength, buffer nature), protein denaturation may be induced by conformation change occurring in the influence of hydrophobic interactions.
Various polystyrene latexes incorporated with acrylamide [3], acrylic acid [5], hydroxyethyl methacrylate [5], N-isopropylacrylamide [11]. Many studies have been devoted to the colloidal characterization of poly (N-isopropylacrylamide) microgel particles, showing their outstanding thermosensitive properties [6-8]. An emulsifier-free polymerization is a versatile technique to have well-defined monodisperse microspheres in various size and surface group functionalities.
The main objective of this work was to examine the adsorption behavior of BSA protein, used here as a model, on to well-characterized NIPA/PEG-EEM copolymer particles. BSA adsorption was investigated as a function of temperature and PEG-EEM content.
Experimental
Materials
N-isopropylacrylamide (NIPA) (Aldrich, USA), poly (ethyleneglycol) ethyl ether methacrylate (PEG-EEM) (Sigma, USA) were used as comonomers. Potassium persulphate (KPS) (BDH, England) was used as initiator. Crosslinking agent was N, N’-methylene bisacrylamide (MBA) (Sigma, USA). All above the reagents were used as purchased. Dispersion medium was distilled water in all experiments (40 ml).
Preparation of polymeric particles
Polymerizations were conducted in an oil-in-water (o/w) system. Typical recipes are listed in Table 1. NIPA, PEG-EEM, MBA and KPS were added to distilled water, in a pyrex glass reactor. Polymerizations were carried out in a constant temperature shaking bath at 65 °C, under nitrogen atmosphere. Polymerization duration was 24 hr. The latex particles were cleaned by washing with water several times to remove the unreacted monomers.
Particle characterization
The average particle size and surface charge density measurements were carried out by Zeta Sizer (Malvern 3000 HSA). FTIR Spectrophotometer (Shimadzu, Model: FTIR-8000 series) with KBr (IR grade)-nanoparticle mixtures in the powder form was employed for structural analysis of the polymer.
BSA adsorption
In adsorption experiments, Bovine Serum Albumin (BSA) (Sigma, U.S.A.) was selected as model protein. Other chemicals used were supplied by BDH (England) and used as received. BSA adsorption on NIPA/PEG-EEM particles was examined in different temperatures (4, 20, 30, 40 °C). BSA concentration was 2 mg/ml. 10 ml of BSA solution prepared in acetate buffer at pH 5. 0.1 g of particle was added to this solution under continous mixing. Particles were interacted with BSA for 2 hours. Adsorption capacity was determined spectrophotometrically (UV/Vis Spectrometer Jasco V-530, Japan). BSA concentration was measured at 280 nm. Amount of BSA interacted with particles were calculated from below formula:
BSA (mg/g particle) = [ X BSA concentration X Volume]/Amount of particle
where A1 and A2 are absorbance of the BSA before and after interaction with particles.
Results and Discussion
Particle preparation
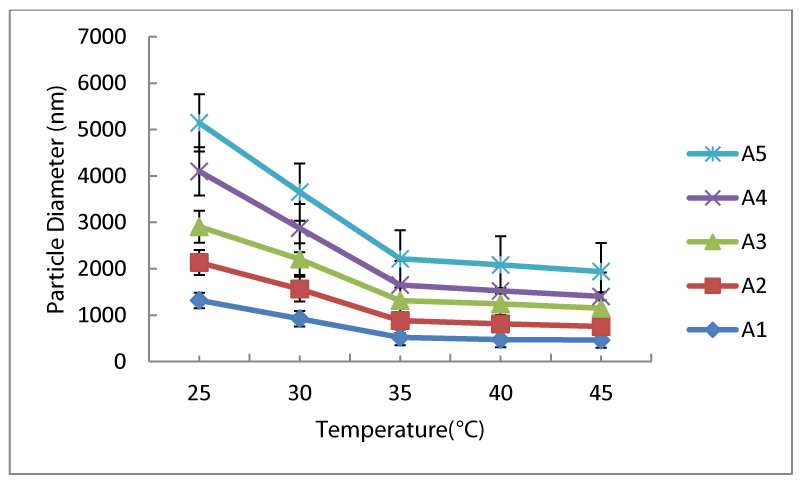
PEG-EEM containing temperature sensitive NIPA nanoparticles in different size range were successfully synthesized by an emulsifier-free emulsion polymerization. PEG-EEM has both hydrophilic PEG and hydrophobic EEM parts in their structure. Therefore, it can behave like surfactant in the aqueous medium. In the first set of experiments, effect of PEG-EEM content on particle size and temperature sensitivity of the particles was examined. For this purpose, PEG-EEM content was changed from 0-0.4 ml in the recipes. At room temperature, particle size decreased from 1300 nm to 800 nm with increased amount of PEG-EEM (0-0.2 ml), however when the content of PEG-EEM reached to 0.2 ml and higher values (0.2-0.4 ml), particle size started to increase as in figure 1. Increase in particle size with decreasing amount of PEG-EEM is reported in literature [12]. As seen in figure 1, all polymeric nanoparticles showed almost the same lower critical solution temperature (LCST), increasing amount of PEG-EEM in the medium does not affect the LCST behavior of the nanoparticles. LCST values of the polymers were around 35°C. Although, at temperatures before LCST, particle size considerably changed with increased amount of PEG-EEM when particles are in their relaxed form, after LCST, particles contracted to their final size and did not change much with increased amount of PEG-EEM in the medium.
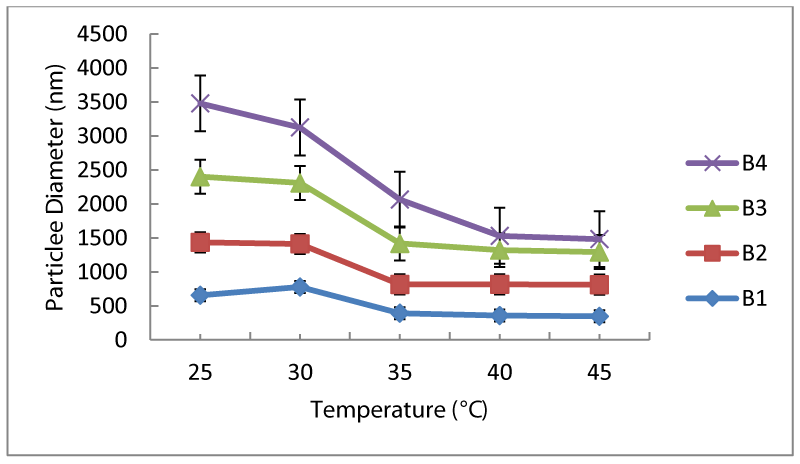
MBA in the recipe was used as crosslinking agent and amount of it was changed from 0.02 to 0.16 g. Particle size increased with the increased MBA content in the recipes (from 650 nm to 1100 nm) as seen in figure 2. Initially, crosslinker amount did not affect the LCST behavior of the polymer as seen in figure 2, however, after addition of 0.16 g, LCST sharply changed from 35°C to 40°C. Sample B4 contracted more with temperature increase compared to the other samples and became 100 nm in size. Increased amount of crosslinker in the medium prevented movement of chains in the free volume as expected.
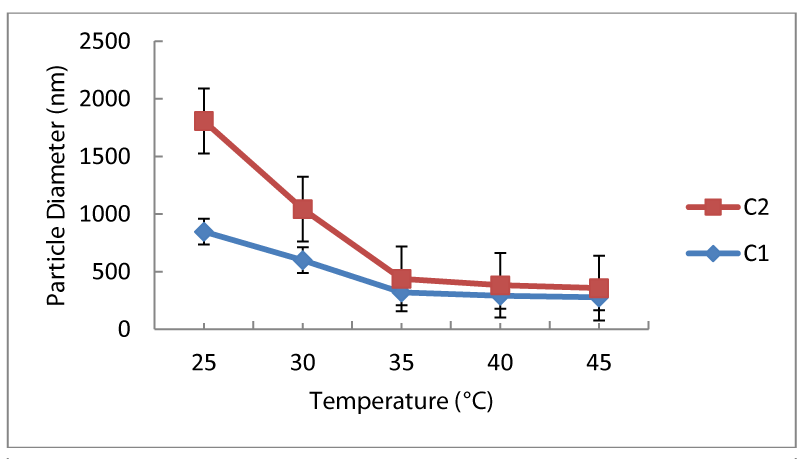
In the third set of experiments, both NIPA and MBA content was decreased to one half of the previous recipes and PEG-EEM content was changed from 0-0.1 g. Particle size measured for the sample without PEG-EEM was around 850 nm, it is increased to 1050 nm with the addition of PEG-EEM in the recipe. In spite of this, sample with PEG-EEM considerably compacted from 1050 nm to 100 nm relative to the sample without PEG-EEM (from 850 nm to 350 nm), with the increase in temperature as seen in figure 3. LCST of both particles, with or without PEG-EEM, was around 35°C and addition of PEG-EEM in the recipe did not change the LCST. After LCST, particles reached their the most compact form and the smallest particle size was measured. Also, it should be noted that the most compacted form of the particles was obtained by PEG-EEM containing samples. Particle size of these samples was below 100 nm after LCST. Therefore, these samples have sponge-like behavior that would be valuable for the further study of adsorption.
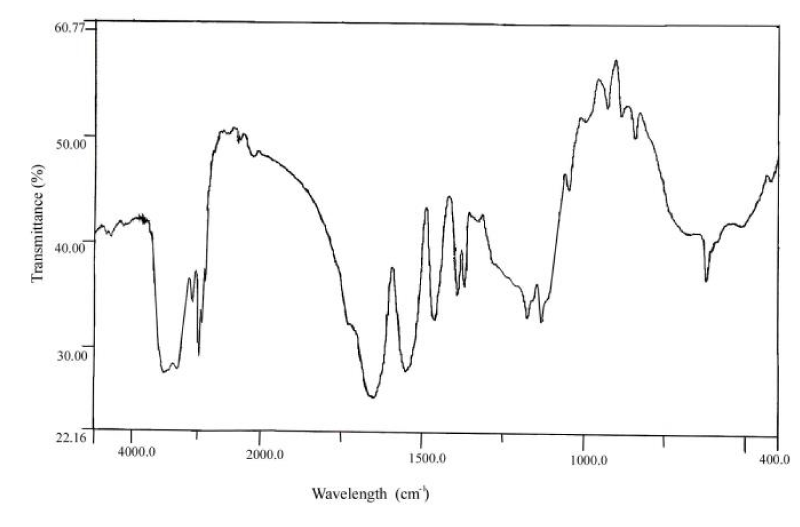
Chemical structure of the particles determined from FTIR spectrum (Figure 4). FTIR spectra of the copolymer synthesized are (KBr Pellet), cm-1: 3500-3400 (w) primary amine (NH stretching), 3078 =CH2, 2976-2878 -CH3 and –CH2, 1725 C=O stretching, 1650 (s) N-H in-plane bending, 1552 (s) N-H stretching, 1460 CH stretching, 1388-1367 CH stretching, 1172, 1130-1049 (w) C-O-C stretch bands, 927-841 (m-w) C-C stretching of main chain, 617 C=O out of plane bends.
Protein adsorption
In recent years, there is a great interest on the design of smart and intelligent polymeric materials for technological applications and fundamental studies [13-21]. These materials can respond with shape and volume changes to small external stimuli, such as temperature, pH, ionic strength, and magnetic fields. Among these intelligent polymeric materials, N-isopropylacrylamide is the most widely studied thermosensitive polymer [22]. Shamim et al studied adsorption and desorption behavior of BSA on surface-modified magnetic nanoparticles covered with thermosensitive polymer (PNIPA) was investigated as a function of temperature, pH, and ionic strength. The results showed that the temperature effect on adsorption/desorption behavior was mainly dependent on the properties of the particles’ surface. The effect of pH was also investigated and it was observed that a smaller amount of protein was adsorbed at higher pH because of the electrostatic repulsive force between protein molecules and latex particles. The maximum amount of protein was adsorbed near the isoelectric point of BSA. Desorption results showed that more protein was desorbed when adsorption was done at lower temperatures and desorption efficiency was found to be higher than 80% [23].
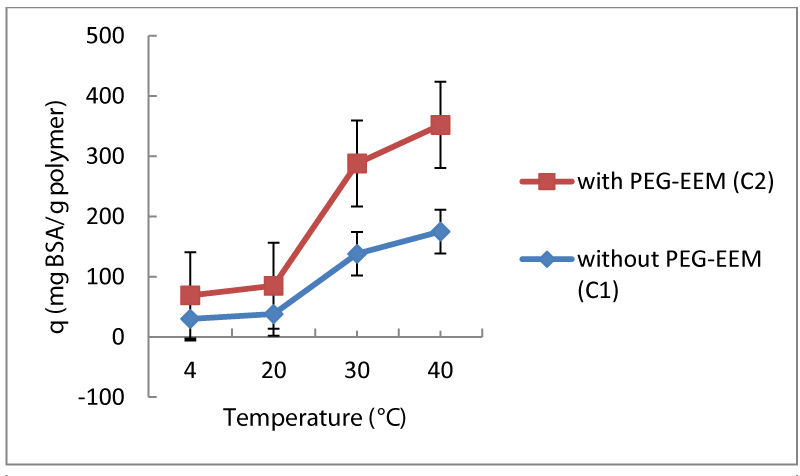
In the adsorption experiments, it is required to have low temperatures to keep the active form of the biological molecules. Temperature sensitive polymers, which have ability to hold an enormous amount of water in their body and have high porosity, are very ideal candidates to become a carrier for biological molecules. In this study, adsorption behavior of both poly (NIPA) and poly(NIPA/PEG-EEM) nanoparticles were comparatively examined to determine the change in adsorption capacity of temperature sensitive latexes in the presence of PEG-EEM in the structure. BSA was selected as model protein and the smallest particles synthesized in the last set of the experiments were used (C coded particles) for the adsorption experiments. First of all, temperature effect on adsorption behavior was examined. In this part, temperature was changed from 4-40°C. It has been known that biological molecules denaturate temperatures above 40°C, so temperatures above 40°C did not included in this study. Interaction of polymeric particle (0.1 g) with 2 mg/ml BSA was realized in pH 5 acetate buffer with gentle mixing for 2 hours. Results obtained can be seen in the figure 5. It was determined that adsorption capacity of nanoparticles without PEG-EEM (sample C1) increased with increased temperature. In the literature, it was reported that adsorption capacity increases with the increase in temperature if there is an interaction between biological molecules in the protein structure and NIPA based polymeric particles [13, 25]. The results we obtained are in very good agreement with literature.
Both addition of PEG-EEM and temperature effect on adsorption capacity of particles examined. In this part of the study, conditions for BSA adsorption kept constant for poly (NIPA/PEG-EEM) as the conditions applied for NIPA alone. Temperature effect on BSA adsorption capacity of NIPA/PEG-EEM based latexes (sample C2) examined in figure 5. Adsorption capacity of both NIPA and NIPA/PEG-EEM latexes increased in similar manner with increase in temperature. However, amount of BSA adsorption on NIPA/PEG-EEM particles was slightly higher than the NIPA alone due to increase in hydrophobicity with addition of PEG-EEM.
It can be concluded that synthesized NIPA/PEG-EEM particles with temperature sensitivity would be an ideal candidate for further protein adsorption studies.
- Galaev IY, Mattiasson B (1999) 'Smart' polymers and what they could do in biotechnology and medicine. Trend Biotechnol 17: 335-340. Link: https://goo.gl/QewHXu
- Hoffman AS, Stayton PS, Bulmus V, Chen G, Chen J, et al. (2000) Founder's Award, Society for Biomaterials. Sixth World Biomaterials Congress 2000, Kamuela, HI,May 15-20, 2000. Really smart bioconjugates of smart polymers and receptor proteins.J Biomed. Mater. Res. 2000, 52,577-586. Link: https://goo.gl/txvZFM
- Schild HG, Prog, Polym (1992) Biomaterials Surface Science. Sci 17:163. Link: https://goo.gl/hgUZwW
- Fang SJ, Kawaguchi H (2002) Colloids and Surfaces A: Physicochem. Eng. Aspects 211: 9.
- Konings BLJC, Pelssers EGM, Verhoeven AJCM, Kamps KMP, Surf BC, et al. (1993) Covalent coupling of antibodies to hydrophilic core-shell particles. Biointerfaces 1: 69-73. Link: https://goo.gl/w5eJJF
- Revilla J, Elaissari A, Carriere P, Pichot C (1996) Polymeric Dispersions: Principles and Applications. J Colloid Interface Sci 180: 405. Link: https://goo.gl/gMi1JM
- Nabzar L, Duracher D, Elaissari A, Chauveteau G, Pichot C, et al. (1998) Colloid Chemistry II. 14: 5062. Link: https://goo.gl/MR27Wb
- Duracher D, Elaissari A, Colloid Polym PC (1999) Microgel Suspensions: Fundamentals and Applications. Sci 277: 905. Link: https://goo.gl/UsZ8BX
- Suzawa T, Shirama H (1991) Adv. Colloid Interface Sci 35: 139.
- Andrade JD, Protein Adsorption (1985) Surface and Interfacial Aspects of Biomedical Polymers, Plenum Link: https://goo.gl/2wJon9
- Pelton RH, Chibante P (1986) Preparation of aqueous latices with N-isopropylacrylamide. Colloid Surf 20: 247-256. Link: https://goo.gl/tuqxzF
- Tuncel A (2000) Emulsion copolymerization of styrene and poly (ethylene glycol) ethyl ethermethacrylate Polymer 41: 1257-1267. Link: https://goo.gl/UXD6Zo
- Shamim N, Hong L, Hidajat K, Uddin MS (2006) Thermosensitive-polymer-coated magnetic nanoparticles: adsorption and desorption of bovine serum albumin J of Colloid and Interface Sci 304:1-8. Link: https://goo.gl/Ekw8Bm
- Nagasaki Y, Ishii T, Uchida K, Otsuka H, Kataoka K (2003) European Cells and Mater 6: 23.
- Valentine MT, Perlman ZE, Gardel ML, Shin JH, Matsudaira P, et al. (2004) Colloid Surface Chemistry Critically Affects Multiple Particle Tracking Measurements of Biomaterials. Biophysical Journal 86: 4004-4014. Link: https://goo.gl/ooUKXX
- Van Vlerken LE, Vyas TK, Amiji MM (2007) Poly (ethylene glycol)-modified Nanocarriers for Tumor-targeted and Intracellular Delivery. Pharmaceutical Research 24: 1405-1414. Link: https://goo.gl/vBFYRC
- Neradovic D, van Nostrum CF, Hennink WE (2001) Thermoresponsive Polymeric Micelles with Controlled Instability Based on Hydrolytically Sensitive N-Isopropylacrylamide Copolymers. Macromolecules 34: 7589-7591. Link: https://goo.gl/mJ1i3R
- Topp MDC, Dijkstra PJ, Talsma H, Feijen J (1997) Thermosensitive Micelle-Forming Block Copolymers of Poly(ethylene glycol) and Poly(N-isopropylacrylamide). Macromolecules 1997, 30: 8518-8520. Link: https://goo.gl/pwqRqC
- Rijcken CJF, Veldhuis TFJ, Ramzi A, Meeldijk JD, van Nostrum CF, et al. (2005) Novel Fast Degradable Thermosensitive Polymeric Micelles Based on PEG-block-poly(N-(2-hydroxyethyl)methacrylamide-oligolactates). Biomacromolecules 6: 2343-2351. Link: https://goo.gl/wXwQDP
- Ramos J, Martin-Molina A, Sanz-Izquierdo MP, Rus A, Borque L, et al. (2003) Amino‐functionalized latex particles obtained by a multistep method: Development of a new immunoreagent. J of Polymer Science: Part A: Polymer Chem 4: 2404-2411. Link: https://goo.gl/KnGNtw
- Zha L, Hu J, Wang C, Fu S, Luo M (2002) The effect of electrolyte on the colloidal properties of poly(N-isopropylacrylamideco-dimethylaminoethylmethacrylate) microgel latexes. Colloid Polym Sci 280: 1116-1121. Link: https://goo.gl/o42vXP
- Duracher D, Veyret R, Elaissari A, Pichot C (2004) Adsorption of bovine serum albumin protein onto amino‐containing thermosensitive core‐shell latexes. Polym Int, 53: 618-626. Link: https://goo.gl/V6LXVv
- Kawaguchi H, Fujimoto K, Mizuhara Y (1992) Hydrogel microspheres III. Temperature-dependent adsorption of proteins on poly-N-isopropylacrylamide hydrogel microspheres. Colloid Polym Sci 270: 53-57. Link: https://goo.gl/mJPNGd
- Fujimoto K, Mizuhara Y, Tamura N, Kawaguchi H (1993) J Intelligent Mater. Syst. Struct 4: 184.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
