Archive of Biomedical Science and Engineering
Nanoderm Extracellular Matrix for Reconstructive Surgery Applications
Pierre Basmaji1, Gabriel Molina de Olyveira2*, Ligia Maria Manzine Costa3, Gino Bruno Francozo1 and José Domingos da Costa Oliveira1
2Department of Physical Chemistry- UNESP/Araraquara-SP, 14800-900, Brazil
3Department of Chemistry- USP/FFCLRP, 14040-901, Brazil
Cite this as
Basmaji P, de Olyveira GM, Manzine Costa LM, Francozo GB, da Costa Oliveira JD (2015) Nanoderm Extracellular Matrix for Reconstructive Surgery Applications. Biomed Sci Eng 1(1): 021-024. DOI: 10.17352/abse.000004Introduction: Bacterial cellulose (BC) can be used in wide area of applied scientific, especially for tissue regeneration and regenerative medicine, lately, bacterial cellulose mats are used in the treatment of skin conditions such as burns and ulcers, because of the morphology of fibrous biopolymers serving as a support for cell proliferation, its pores allow gas exchange between the organism and the environment. Moreover, the nanostructure and morphological similarities with collagen make BC attractive for cell immobilization, cell support and Natural Extracellular Matrix (ECM) Scaffolds. In this scope, Natural ECM is the ideal biological scaffold since it contains all the components of the tissue. The development of mimicking biomaterials and hybrid biomaterial can further advance directed cellular differentiation without specific induction.
Methods: The acetic fermentation process was achieved by using glucose as a carbohydrate source. Results of this process are vinegar and a nanobiocellulose biomass. The modifying process is based on the addition of hyaluronic acid and chondroitin sulfate (1% w/w) to the culture medium before the bacteria is inoculated. After, vegetal stem cells were added in the system, which were chosen because has wound healing properties that help new skin formation. (Casearia Sylvestris).
Result: Bacterial cellulose (Nanoskin®) was successfully modified by changing the fermentation medium as shown by FTIR and TGA, which produced necessary materials for regenerative medicine. Nanoskin Natural extracellular matrix (ECMs) perform the tasks necessary for tissue formation, maintenance, regulation and function, providing a powerful means of controlling the biological performance of regenerative materials.
Conclusion: Nanoderm® Natural extracellular matrix (ECMs) perform the tasks necessary for tissue formation, maintenance, regulation and function, providing a powerful means of controlling the biological performance of regenerative materials. Understanding how cells interact with these to assemble their own ECM and how the scaffolds can be used to control delivery of signals in a temporal and spatial manner to guide or maintain cell differentiations need future investigation. But, undoubtedly, natural-origin polymers or nature-inspired materials appear as the natural and desired choice for medical applications.
Introduction
Bacterial cellulose (BC) can be used in several areas of applied scientific, especially for tissue regeneration and regenerative medicine, lately, bacterial cellulose mats are used in the treatment of skin wound healing such as burns and ulcers, because of the morphology of fibrous biopolymers serving as a support for cell proliferation, its pores allow gas exchange between the organism and the environment [1-3].
Tissue engineering is a recent field that create functioning artificial tissues and organs. Major considerations in tissue engineering include both the type of cell and the substrate (scaffold) to be used. Many strategies use an artificial scaffold that functions as the ECM to facilitate both organization and differentiation of implanted cells into a functional 3D tissue [4].
In this scope, Natural ECM is the ideal biological scaffold since it contains all the components of the tissue. Constructive remodeling can be performed using such natural ECM scaffolds and vegetal/animal stem cells since, the cells can be delivered to the site of infraction and once delivered the cells adhere and are not “lost”. The development of niche mimicking biomaterials and hybrid biomaterial can further advance directed differentiation without specific induction [5,6].
The extracellular matrix (ECM) contains an abundant variety of signals that are received by cell surface receptors and contribute to cell adhesion and cell fate, via regulation of cellular activities such as proliferation, migration and differentiation. As such, regenerative medicine studies often rely on mimicking the natural ECM to promote the formation of new tissue by host cells, and characterization of natural ECM components is vital for the development of new biomimetic approaches [7,8].
Bacterial cellulose (Nanoskin®) is a natural cellulose produced by bacterial synthesis by biochemical steps and self-assembling of the secreted cellulose fibrils on the medium. Shaping of BC materials in the culture medium can be controlled by the type of cultivation that changes chain size, origin of strains which produces different proportions of crystalline phase of BC and the kind of bioreactor. BC hydrogel or BC in dry state is then obtained by methods, such as freeze-drying [9,10]. The structural features of microbial cellulose, its properties and compatibility as a biomaterial for regenerative medicine can be changed by modifying its culture medium [11], or surface modification by physical [12,13], chemical methods[14] and genetic modifications [15], to obtain a biomaterial with less rejection when in contact to the body [16,17].
Bacterial cellulose fibers (Nanoskin®) mimics Collagen in creating an extra cellular matrix in the wound, which is neither originating from Animals (e.g. SIS matrix) nor synthetic (man-made), and it therefore must be described as Artificial Biology. This artificial biological ECM replaces the body’s own lost or damaged ECM and also stimulates the body to produce more of its own Collagen, which supports the body’s wound healing closure mechanism and stimulating Fibroblast production and subsequently TGF –b production [18]. So, granulation and epithelialization will start due to the presence of fibroblasts, endothelial cells are attracted to the wound producing growth factors. The combination of collagen and fibronectin forms the new ECM, ECM synthesis and new vessels, granulation tissue formation and epithelialization by Keratinocyte migration, results in and increase in the dermis volume and accelerating the healing [19]. Besides, fibers also activate NK killer cells (activates T and B cells), helping to balance the wound. Body understands when produce positive items (collagen for example and when stop). Correcting wound modulation and starting correct cellular communication, body then auto regulates the delivery of the necessary components necessary to promote wound repair [20-22].
In this work, novel studies of natural nanocomposites with bacterial cellulose (Nanoderm®) for functional materials are reported. In order to produce scaffolds with drug delivery ability, porous structure and better cell adhesion, fermentation changes in gel bacterial cellulose with chondroitin sulfate, hyaluronic acid and vegetal stem cells were performed for mimicking ECM to cells support and built new material for wound healing.
Materials and Methods
Materials
The bacterial cellulose raw material was provided from Innovatec´s (São Carlos SP, Brazil). Chondroitin sulfate and hyaluronic acid sodium salt from Streptococcus equi (bacterial glycosaminoglycan polysaccharide) were purchased from Sigma Aldrich. Vegetal stem cells were obtained from Brazilian environment, Casearia Sylvestris.
Methods
Synthesis of Bacterial Cellulose and bacterial cellulose/chondroitin sulfate/ hyaluronic acid: The acetic fermentation process was achieved by using glucose as a carbohydrate source. Results of this process are vinegar and a nanobiocellulose biomass. The modifying process is based on the addition of hyaluronic acid and chondroitin sulfate (1% w/w) to the culture medium before the bacteria is inoculated. Bacterial cellulose (BC) is produced by Gram-negative bacteria Gluconacetobacter xylinus, which can be obtained from the culture medium in the pure 3-D structure, consisting of an ultra-fine network of cellulose nanofibers [19].
Vegetal Stem cells: The material of the plant of interest is collected and induced damage to causing the formation of scar tissue called callus. This tissue consists of totipotent cells, undifferentiated (stem cells) are collected and grown on agar plates for Microbiology-Fluka (Petri dish that contains culture medium-typically agar plus nutrients-Trypticase soy agar) to complete differentiation and generation of a homogeneous culture (2-10 days).
Cultures of these stem cells are grown in bioreactors [(temperature 25°C, pH=6,8 and culture medium with carbon source(glucose) and nitrogen source( tea)] and the batch is collected after all the sugar was metabolized. The cells are washed and homogenized to release secondary metabolites. Soluble metabolites in oil and water are collected and, if you need the isomalt -based spraying is performed. Vegetal stem cells (Casearia Sylvestris) were chosen because has wound healing properties that help new skin formation.
Bionanocomposite preparation
In the present study, a novel biomaterial has been explored and different bacterial cellulose nanocomposites have been prepared; 1) BC/chondroitin sulfate and hyaluronic acid. Samples were washed and it´s medium was changed with stem cells culture medium as illustrated in Figure 1.
Characterization
Transmission infrared spectroscopy (FTIR, Perkin Elmer Spectrum 1000): Influences of hyaluronic acid and chondroitin sulfate in bacterial cellulose were analyzed in the range between 250 and 4000 cm-1 and with 2 cm-1 resolution with samples.
Thermo gravimetric analysis (TGA): Thermo gravimetric analysis (TGA) was carried out for biocomposites using a NETZSCH TG 209F1 in oxygen environment, with a heating rate of 10C/min. The temperature range scanned was from 25 Celsius degree to 650 Celsius degree. The weight of all specimens was maintained around 10 mg.
In vivo analysis- Evaluation: In vivo analysis- Evaluation- Clinical study of four weeks at Nanoskin-Biotechnology Research and Development, São Carlos -SP. Evaluation model - wound in a old man, admitted with cancer lesion in Maxilla (5X4mm).
Results and Discussion
FTIR
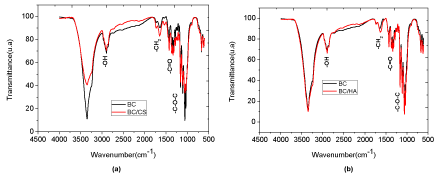
Interaction between bacterial cellulose with hyaluronic acid and chondroitin sulfate Influences of hyaluronic acid(HA) and chondroitin sulfate(CS) in bacterial cellulose were analyzed in the range between 250 and 4,000 cm-1 and with resolution of 2 cm-1 with FTIR analysis. The main features of the bacterial cellulose in infrared spectroscopy is: 3,500 cm-1: OH stretching, 2,900 cm-1: CH stretching of alkane and asymmetric CH2 stretching, 2,700 cm-1: CH2 symmetric stretching, 1,640 cm-1: OH deformation, 1,400 cm-1: CH2 deformation, 1,370 cm-1: CH3 deformation, 1,340 cm-1: OH deformation and 1,320-1,030 cm-1: CO deformation [23].
In the case of FT-IR spectra of bacterial cellulose/chondroitin sulfate (BC/CS) nanocomposites, the intensity of sharp absorption band at 3349 cm-1 has increased significantly with a wider profile, indicating that the hydrogen-bonding strengths in these compounds have been reduced, since the hydrogen bonds between cellulose molecules are interfered by CS molecules (Figure 2a). There were no shifts in the bands of carboxylate 1640 cm-1 and sulfate groups that appear at 1250 cm-1 and 1230 cm-1 in bacterial cellulose/chondroitin sulfate nanocomposites (Figure 2a). However, it has exhibited broad overlapping bands at 1640 cm-1 (primary amide bond stretching) and 1564 cm-1 (aromatic C=C stretching vibrations), as well as N-H bending vibrations at 1508 cm-1 [24]. Besides, it was assigned an increase of intensity absorption bands at 1250 cm-1 and 1230 cm-1 due to sulphate-related modes, corresponding to antisymmetric and symmetric stretching of the sulphate group, respectively. The intensity of the antisymmetric bridge oxygen stretching band at 1163 cm-1 was reduced after formation of BC/CS nanocomposite, indicating a change in the hydrogen-bonding of the bridge oxygen after the addition of chondroitin sulfate in the system. The visible spectral profile changes observed at 897 cm-1, corresponding to characteristic of β-anomers or β-linked glucose polymers, assigned as C-O-C stretching of the β-(1→4)-glycosidic linkage. This band becomes sharp and strong in the BC/CS nanocomposite. It can be explained from participation of the oxygen atom attached to C1 in this vibration and changes in the hydrogen bonding in cellulose [23]. Therefore, the results clearly show one possible interaction between bacterial cellulose and chondroitin sulfate, mainly by hydrogen interactions between hydroxyl and carbonyl groups.
It can be observed similar OH stretching (at 2,900 cm-1) in bacterial cellulose/hyaluronic acid nanocomposites (BC/HA), mainly because of the NH2 interaction with hydroxyl groups (Figure 2b). Besides, it can be observed a shift from (H-O-H) absorption band at 1640 cm-1 of bacterial cellulose structures and amide I absorption from HA at 1620 cm-1, indicating an integrated HA/BC molecules. Another absorption peak was obtained in the range of 1490 cm−1 on both samples, which shows the presence of a carbonyl group in the bacterial cellulose together with bonds corresponding to those of glycoside, including C–O–C at 1162 cm−1 (as in the case of natural cellulose) [23]. These results clearly show one possible interaction between bacterial cellulose and hyaluronic acid, mainly by hydrogen interactions between hydroxyl and carbonyl groups.
TGA
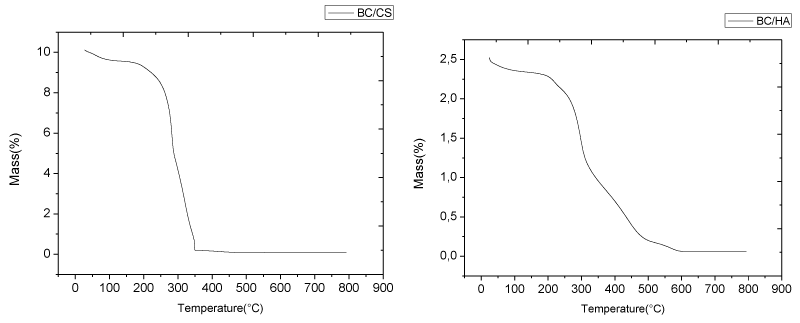
In order to analyze thermal behavior for bionanocomposites are characterized typical weight loss verses temperature plots. The TG spectrum (Figure 3) shows a weak loss of weight due to the evaporation of water (at temp. 85 celsius) and also quick drop in weight at a temperature of approx. 300 celsius is mainly attributed to thermal depolymerization of cellulose and the cleavage of glycosidic linkages of cellulose [24,25], complete degradation of cellulose take place between 275 and 400 celsius [26,27].
All system has similar thermal behavior in bacterial cellulose showed significant alterations. A carbonaceous residue was similar in BC membranes, around 0% at 600 celsius, however sample with hyaluronic acid has little differences in thermal behavior than tested others mainly because there is higher hydrogen bond between bacterial cellulose groups (hydroxyl) and hyaluronic acid (acetyl) which changes bacterial cellulose fibers formation and thermal properties.
In vivo analysis- Nanoderm®
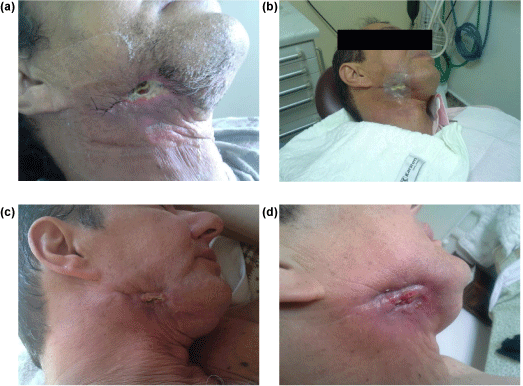
Patient after surgical act in 03-27-2015, received in surgical area Rayon wound dressing covered with Rifocine. This wound dressing protect surgical area and helps clotting, decreasing pain because block food and drink sediment as illustrted in Figure 4a.
However, because patient has pain yet, it was tested Nanoderm® wound dressing as illustrated in Figure 4b in 04-10-2015.
After cover surgical area with Nanoderm® in the core, patient report pain decrease after first Nanoderm® wound dressing changes like illustrated in Figure 4c, and after each change it was improve wound healing with new re-epithelialization as illustrated in Figure 4d. It was take 2 Nanoderm® wound dressing changes and after four months of Nanoderm® uses, it can be observed complete re-epithelialization and wound healing as illustrated in Figure 4d.
Conclusion
Bacterial cellulose (Nanoderm®) was successfully modified by changing the fermentation medium as shown by FTIR and TGA, which produced necessary materials for regenerative medicine. Nanoderm® Natural extracellular matrix (ECMs) perform the tasks necessary for tissue formation, maintenance, regulation and function, providing a powerful means of controlling the biological performance of regenerative materials. However, understanding how the scaffolds can be used to control delivery of signals in a temporal and spatial manner to guide or maintain cell differentiations need future investigation. But, undoubtedly, natural-origin polymers or nature-inspired materials appear as the natural and desired choice for medical applications.
UNESP-IQ-Chemistry Institute; Innovatec´s-Biotechnology Research and Development.
- Rana D, Zreiqat H, Jessel NB, Ramakrishna S, Ramalingam M (2015) Development of decellularized scaffolds for stem cell-driven tissue engineering. J Tissue Eng Regen Med 10.1002.
- Prestwich GD, Healy KE (2015) why regenerative medicine needs an extracellular matrix Expert. Opin Biol Ther 15: 3-7 .
- Mudla A (2014) Tendon cell behavior and regeneration Effects of extracellular matrix and substrate stiffness. Journal of Purdue Undergraduate Research 4: 10.5703 .
- Volpato FZ, Führmann T, Migliaresi C, Hutmacher DW, Dalton PD (2013) Using extracellular matrix for regenerative medicine in the spinal cord. Biomaterials 34: 4945-4955 .
- Rahman GA, Adigun IA, Fadeyi A (2010) Epidemiology, etiology, and treatment of chronic leg ulcer experience with sixty patients. Annals of African Medicine 9: 1-4.
- Basmaji P, Olyveira GM, Costa LMM, Souza JAM, Lucchesi GL, et al. (2015) Natural ECM-Biological Wound Dressing. Aperito J. Nanosci. Technol 2: 105 .
- El-Hoseny SM, Basmaji P, Olyveira GM, Costa LMM, Alwahedi AM, et al. (2015) Natural ECM-Bacterial Cellulose Wound Healing – Dubai Study. Journal of Biomaterials and Nanobiotechnology 6: 237-246 .
- Han A, Zenilman JM, Melendez JH, Shirtliff ME, Agostinho A, et al. (2011) The importance of a multi-faceted approach to characterizing the microbial flora of chronic wounds. Wound Repair and Regeneration 19: 532-541 .
- Costa LMM, Olyveira GM, Basmaji P, Valido DP, Góis PBP, et al. (2012) Novel Otoliths/Bacterial Cellulose Nanocomposites as a Potential Natural Product for Direct Dental Pulp Capping. J Biomater Tissue Eng 2: 48-53 .
- Olyveira GM, Santos ML, Riccardi CS, Costa LMM, Daltro PB, et al. (2015) Bacterial cellulose biocomposites for periodontology treatment. Adv Sci Eng Med 7: 409-414 .
- Costa LMM, Olyveira GM, Basmaji P, Filho LX (2011) Bacterial Cellulose towards Functional Green Composites Materials. J Bionanosci 5: 167-172 .
- Costa LMM, Olyveira GM, Basmaji P, Filho LX (2012) Bacterial Cellulose Towards Functional Medical Materials. J. Biomater. Tissue Eng 2: 185-196 .
- Olyveira GM, Costa LMM, Basmaji P (2013) Physically Modified Bacterial Cellulose as Alternative Routes for Transdermal Drug Delivery. J. Biomater. Tissue Eng 3: 227-232 .
- Olyveira GM, Riccardi CS, Santos ML, Costa LMM, Daltro PB, et al. (2014) Bacterial cellulose nanobiocomposites for periodontal disease.J. Bionanosci 8: 319-324 .
- Ramalingam M, Rana D (2015) Impact of Nanotechnology in Induced Pluripotent Stem Cells-driven Tissue Engineering and Regenerative Medicine. J Bionanosci 9: 13-21 .
- Filho LX, OLyveira GM, Costa LMM, Basmaji P (2013) Novel electrospun nanotholits/PHB scaffolds for bone tissue regeneration J. Nanosci. Nanotechnol 13: 4715-4719 .
- Olyveira GM, Costa LMM, Basmaji P, Filho LX (2011) Bacterial Nanocellulose for medicine regenerative . J Nanotech Eng Med 2: 34001 .
- Olyveira GM, Santos ML, Daltro PB, Basmaji P, Daltro GC, et al. (2014) Bacterial cellulose/chondroitin sulfate for dental materials scaffolds. J. Biomater. Tissue Eng 4: 150-154 .
- Olyveira GM, Santos ML, Costa LMM, Daltro PB, Basmaji P, et al. (2014) Bacterial Cellulose Nanobiocomposites for Dental Materials Scaffolds. J. Biomater. Tissue Eng 4: 536-542 .
- Olyveira GM, Santos ML, Costa LMM, Daltro PB, Basmaji P, et al. (2014) Bacterial biocomposites for guided tissue regeneration. Sci. Adv. Mater 6: 2673-2678 .
- Olyveira GM, Santos ML, Riccardi CS, Costa LMM, Daltro PB, et al. (2015) Physically Modified Bacterial Cellulose Biocomposites for Guided Tissue Regeneration. Sci. Adv. Mater 7: 1657-1664 .
- Olyveira GM, Riccardi CS, Santos ML, Costa LMM, Daltro PB, et al. (2015) Physically modified bacterial cellulose biocomposites for dental materials scaffolds. Materials Focus 4: 111-117 .
- Salmen L, Akerholm M, Hinterstoisser B (2005) Two-dimensional Fourier transform infrared spectroscopy applied to cellulose and paper. Marcel Dekker 159-187 .
- Manfredi LB, Rodriguez ES, Wladyka-Przybylak M, Vazquez A (2006) Thermal degradation and fire resistence of unsaturated polyester modified acrylic resins and their composites with natural fibers. Polym Degrad Stab 91: 255-261 .
- Ouajai S, Shanks RA (2006) Solvent and enzyme induced recrystallization of mechanically degraded hemp cellulose. Cellulose 13: 31-44 .
- Alvarez VA, Ruseckaite RA, Vazquez A (2006) Degradation of sisal fibre/mater Bi-Y biocomposites buried in soil. Polym Degrad Stab 91: 3156-3162 .
- Deepa B, Abraham E, Cherian BM, Bismarc A, Blaker JJ, et al. (2011) Structure, morphology and thermal characteristics of banana nano fibers obtained by steam explosion. Bioresour Technol 102: 1988-1987 .
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
