Annals of Bone Marrow Research
Experimental in vitro modeling of hematopoiesis by using diffusion chambers of an original design
Denys Bilko*
Associate Professor, Department of Laboratory diagnostics of Biological Systems, National University of “Kyiv-Mohyla Academy” Kyiv, Ukraine
*Corresponding author: Dr. Denys Bilko, Associate Professor, Department of Laboratory diagnostics of Biological Systems, National University of “Kyiv-Mohyla Academy” Kyiv, Ukraine, E-mail: comraduk@yahoo.co.uk
DOI: 10.17352/abmr.000009
Received: 14 July, 2022 | Accepted: 22 July, 2022 | Published: 23 July, 2022
Keywords: Culture in vitro; Hematopoiesis; Hematopoietic stem cell; Hematopoietic cell expansion; Gel diffusion chambers; Feeder layers
Cite this as
Suriu C, Braester A, Barhoum M (2022) Experimental in vitro modeling of hematopoiesis by using diffusion chambers of an original design. Ann Bone Marrow Res 6(1): 007-008. DOI: 10.17352/abmr.000009
Copyright Licence
© 2022 Suriu C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
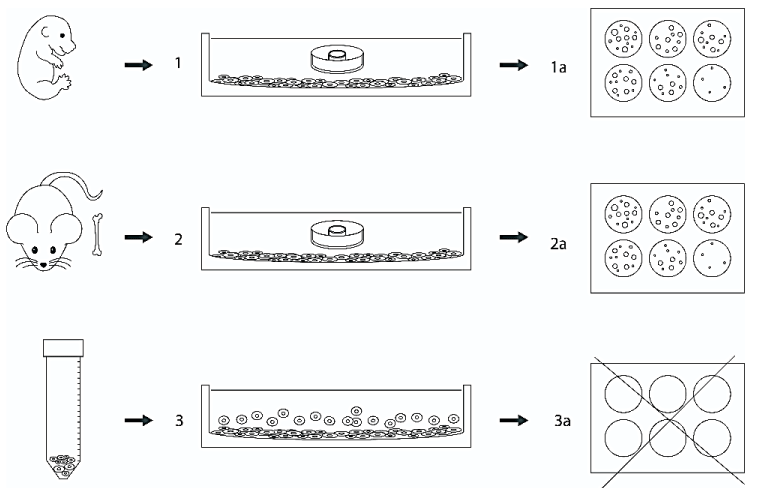
One of the most important tasks of biotechnology and regenerative medicine is to achieve long-term expansion of hematopoietic stem cells. One of the promising approaches to optimizing the cultures of hematopoietic cells is the creation of a microenvironment in the form of feeder stromal layers, which allows for the reproduction of the combination of soluble cytokines and growth factors necessary for hematopoiesis and promotes an increase in their proliferation [1,2]. However, there is always a risk of contamination of the future graft by feeder cells. Therefore, the purpose of the work was to create a model for supporting hematopoiesis in soft hydrogel diffusion chambers immersed in a nutrient medium with a feeder layer and to determine whether direct contact between the hematopoietic cells and the feeder is necessary for full support of hematopoiesis in vitro. To realize this goal (see Figure 1), the method of culturing in gel diffusion chambers in vitro, clonogenic assay in semiliquid agar, cytological, immunohistochemical methods, and methods of variation statistics were used. AC133+ cells isolated from cord blood were injected into the inner cavity of polyacrylamide gel chambers and transferred to Nunc six-well plastic mattresses with pre-prepared feeder layers, at the rate of 1 chamber per 1 well. The feeder layers from mouse embryonic feeders (MEF) or femur bone of an adult mouse were used; after the formation of the monolayer in vitro, proliferation was stopped using Mitomycin-C. The gel diffusion chambers immersed in the nutrient medium were in the state of a “submarine”, which does not rise to the surface and does not touch the feeder layer (due to the selected gel stiffness) but is located submerged in the nutrient medium [3].
The colony-forming capacity of hematopoietic progenitor cells cultured on substrates from both sources was determined using a semisolid agar culture method (Difco, USA) and it was concluded that the presence of a feeder layer from both sources promotes cell proliferation in the diffusion chambers. Despite the morphological similarity of the cells of the feeder monolayers belonging to the category of fibroblast-like cells, they turned out to be different in functional properties. Thus, the efficiency of colony formation of feeders from adult mouse cells was 48.7 ± 3.5, and on the MEF feeders - 68.50 ± 5.8, that is, the colony-forming capacity was higher in the second case. This means that the feeder layer from the embryonic tissue provides greater colony-forming activity than the feeder from the tissue of an adult animal due to its greater ability to support hematopoiesis. The difference between the indicators is statistically significant (p<0.05). Meanwhile assessment of colony formation in the case when hematopoietic cells contact with feeder layers is complicated or impossible because of contamination of suspension by feeder cells. The number of clusters did not differ significantly in the specified terms of cultivation. This can be explained by the fact that clusters are formed from more mature precursors that have undergone several divisions before entering the culture and cannot fully reflect the processes of hematopoiesis. The results of culturing hematopoietic cells in gel diffusion chambers immersed in the medium above the feeder layer showed the adequacy of such a model for the study of cellular and humoral interactions. In addition, the high colony-forming activity of hematopoietic progenitor cells in feeder cultures indicates that cytokines and growth factors produced by feeder stromal cells are sufficient to remotely support hematopoiesis, and direct contacts between hematopoietic cells and the stromal matrix are not necessary for successful long-term hematopoiesis in vitro. In the future, the model can be used in regenerative medicine to obtain potential hematopoietic stem cell grafts without the risk of contamination by feeder cells and to study the effect of different feeder layers, cytokines, and growth factors on the development of remotely cultured hematopoietic stem cells.
- Bilko D, Chaikovsky YBY. The role of substrate stiffness in maintaining pluripotecy of embryonic stem cells in vitro culture. ISSN 0201-8489. Phyziol. journ., – 2021. – Vol. 67, No. 3. – P. 27–34. – https://doi.org/10.15407/fz67.03.027.
- De Wynter E, Ploemacher RE. Assays for the Evaluation of Human Haematopoietic Stem Cells // J. Biol. Regulation Homeost Agents - 2001. - Vol.15. No. 1. - P.23-27.
- Congrains A, Bianco J, Rosa RG, Mancuso RI, Saad STO. 3D Scaffolds to Model the Hematopoietic Stem Cell Niche: Applications and Perspectives. Materials (Basel, Switzerland). 2021 Jan;14(3): 569. doi: 10.3390/ma14030569 PMID: 33530372 PMCID: PMC7865713
Cite this as
Suriu C, Braester A, Barhoum M (2022) Experimental in vitro modeling of hematopoiesis by using diffusion chambers of an original design. Ann Bone Marrow Res 6(1): 007-008. DOI: 10.17352/abmr.000009Copyright Licence
© 2022 Suriu C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.One of the most important tasks of biotechnology and regenerative medicine is to achieve long-term expansion of hematopoietic stem cells. One of the promising approaches to optimizing the cultures of hematopoietic cells is the creation of a microenvironment in the form of feeder stromal layers, which allows for the reproduction of the combination of soluble cytokines and growth factors necessary for hematopoiesis and promotes an increase in their proliferation [1,2]. However, there is always a risk of contamination of the future graft by feeder cells. Therefore, the purpose of the work was to create a model for supporting hematopoiesis in soft hydrogel diffusion chambers immersed in a nutrient medium with a feeder layer and to determine whether direct contact between the hematopoietic cells and the feeder is necessary for full support of hematopoiesis in vitro. To realize this goal (see Figure 1), the method of culturing in gel diffusion chambers in vitro, clonogenic assay in semiliquid agar, cytological, immunohistochemical methods, and methods of variation statistics were used. AC133+ cells isolated from cord blood were injected into the inner cavity of polyacrylamide gel chambers and transferred to Nunc six-well plastic mattresses with pre-prepared feeder layers, at the rate of 1 chamber per 1 well. The feeder layers from mouse embryonic feeders (MEF) or femur bone of an adult mouse were used; after the formation of the monolayer in vitro, proliferation was stopped using Mitomycin-C. The gel diffusion chambers immersed in the nutrient medium were in the state of a “submarine”, which does not rise to the surface and does not touch the feeder layer (due to the selected gel stiffness) but is located submerged in the nutrient medium [3].
The colony-forming capacity of hematopoietic progenitor cells cultured on substrates from both sources was determined using a semisolid agar culture method (Difco, USA) and it was concluded that the presence of a feeder layer from both sources promotes cell proliferation in the diffusion chambers. Despite the morphological similarity of the cells of the feeder monolayers belonging to the category of fibroblast-like cells, they turned out to be different in functional properties. Thus, the efficiency of colony formation of feeders from adult mouse cells was 48.7 ± 3.5, and on the MEF feeders - 68.50 ± 5.8, that is, the colony-forming capacity was higher in the second case. This means that the feeder layer from the embryonic tissue provides greater colony-forming activity than the feeder from the tissue of an adult animal due to its greater ability to support hematopoiesis. The difference between the indicators is statistically significant (p<0.05). Meanwhile assessment of colony formation in the case when hematopoietic cells contact with feeder layers is complicated or impossible because of contamination of suspension by feeder cells. The number of clusters did not differ significantly in the specified terms of cultivation. This can be explained by the fact that clusters are formed from more mature precursors that have undergone several divisions before entering the culture and cannot fully reflect the processes of hematopoiesis. The results of culturing hematopoietic cells in gel diffusion chambers immersed in the medium above the feeder layer showed the adequacy of such a model for the study of cellular and humoral interactions. In addition, the high colony-forming activity of hematopoietic progenitor cells in feeder cultures indicates that cytokines and growth factors produced by feeder stromal cells are sufficient to remotely support hematopoiesis, and direct contacts between hematopoietic cells and the stromal matrix are not necessary for successful long-term hematopoiesis in vitro. In the future, the model can be used in regenerative medicine to obtain potential hematopoietic stem cell grafts without the risk of contamination by feeder cells and to study the effect of different feeder layers, cytokines, and growth factors on the development of remotely cultured hematopoietic stem cells.
- Bilko D, Chaikovsky YBY. The role of substrate stiffness in maintaining pluripotecy of embryonic stem cells in vitro culture. ISSN 0201-8489. Phyziol. journ., – 2021. – Vol. 67, No. 3. – P. 27–34. – https://doi.org/10.15407/fz67.03.027.
- De Wynter E, Ploemacher RE. Assays for the Evaluation of Human Haematopoietic Stem Cells // J. Biol. Regulation Homeost Agents - 2001. - Vol.15. No. 1. - P.23-27.
- Congrains A, Bianco J, Rosa RG, Mancuso RI, Saad STO. 3D Scaffolds to Model the Hematopoietic Stem Cell Niche: Applications and Perspectives. Materials (Basel, Switzerland). 2021 Jan;14(3): 569. doi: 10.3390/ma14030569 PMID: 33530372 PMCID: PMC7865713
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
