Archives of Anatomy and Physiology
Postnatal Changes in the Morphology of the Myocardium in Rat Ventricles
Georgi Kotov1, Alexandar Iliev1*, Boycho Landzhov1, Lazar Jelev1, Iva N. Dimitrova2, Dimka Hinova-Palova1
2Department of Cardiology, University Hospital “St. Ekaterina”, Medical University of Sofia, Bulgaria
Cite this as
Kotov G, Iliev A, Landzhov B, Jelev L, Dimitrova IN, et al. (2017) Postnatal Changes in the Morphology of the Myocardium in Rat Ventricles. Arch Anat Physiol 2(1): 011-017. DOI: 10.17352/aap.000005Background: Aging of the myocardium is a dynamic process which involves progressive loss of cardiomyocytes due to necrosis and apoptosis, interstitial fibrosis and reactive hypertrophy of the remaining vital cardiomyocytes. In our study, we investigated the postnatal changes in the myocardium of 15 adult male Wistar rats, distributed in the following age groups: 2 weeks, 1, 3, 6 and 12 months old. We used routine hematoxylin and eosin staining and Mallory’s trichrome stain.
Results: Hypertrophy of the cardiomyocytes and accumulation of extracellular matrix proteins in the interstitial space in the aging myocardium were observed. The occurrence of these processes was more dynamic in the left ventricle as opposed to the right ventricle owing to the functional dissimilarities between the two ventricles and a more pronounced effect of afterload on the left ventricle.
Conclusions: In conclusion, it can be stated that aging of the myocardium is a dynamic process, characterized by hypertrophy of the cardiomyocytes, reduced capillary density and increased deposition of collagen.
Introduction
Comprehensive studying of the myocardial architecture is of key importance for the understanding of the precise mechanisms of cardiac function and the pathogenetic processes which form the basis of cardiovascular disorders. Multiple contemporary studies reveal the participation of the myocardium in virtually every disease - either as a primary site of the pathophysiological changes or as an affected organ secondary to the pathological process. It is true that the myocardium possesses a remarkable diagnostic and therapeutic potential. Studying of the arrangement and the specific characteristics of the cardiac muscle cells can provide a morphological basis for the diagnostics of various disorders of the heart. The present study reflects our efforts to understand the morphology of cardiomyocytes and the myocardium as a whole in hearts from male rats from different periods of the postnatal development and to elucidate the importance of morphological differences between the left and the right ventricle. To do so, we used different staining techniques which allowed us to clearly observe sections of the cardiac muscle cells in different planes and changes in the myocardial structure. Although our studies were conducted on experimental animals, the environment in which the cardiomyocytes existed was identical to the conditions in the human body and complied with the universal biological principles.
The cardiomyocyte, or the cardiac muscle cell, is a cylindrically shaped cell with approximate diameter 10 – 15 µm and length of about 100 µm [1]. Their mean diameter is intermediate as compared to that of the small atrial cells and the wider Purkinje fibers. On light microscopy the cardiomyocyte appears as a cross-striated cell and has one or two centrally positioned, slightly elongated nuclei with low density [1,2]. Muscle fibers in the ventricles are located in very close proximity to one another, thus forming wide bundles of branching cells, which more often than not connect in longitudinal direction through specialized structures known as intercalate discs. Inside, the ventricular cardiomyocyte is filled with alternating chains of sarcomeres and mitochondria [3]. An often encountered pattern of alignment is in a Y-shape, with the participation of three Purkinje fibers. There are also zones with mosaic alignment of the cells. This type of cellular organization, as well as the presence of a complex intercalate disc play a key role in the rapid conduction between the Purkinje fibers [3].
The alignment of the cardiomyocytes leads to the formation of a network or a functional syncytium which represents the main building unit of the myocardium. Other cells are also present in the structure of the myocardium: fibroblasts, endothelial cells and smooth muscle cells [4,5]. The connective tissue surrounding the cardiomyocyte includes the following three layers, listed herein starting from the outermost layer: epimysium, perimysium and endomysium. The normal extracellular matrix is produced by the myocardial fibroblasts as well as by the cardiomyocytes themselves. It is built of collagen types I, III and IV, laminin, fibronectin and proteoglycans. It seems that it plays a key role in the formation of the myofibrils and the intercalate discs during the differentiation and maturation of the myocardial cells from cardiac stem cells [6,7].
The aim of the present study was to elucidate the specifics of the normal morphology of the individual cardiomyocytes and the myocardium as a whole during different periods of the postnatal development and to compare the nature of the changes in myocardial structure between the left and the right ventricle.
Materials and Methods
Fifteen adult male Wistar rats, distributed in the following age groups: 2 weeks, 1, 3, 6 and 12 months old, were used for this study.
The animals were anesthetized with ether and cervical dislocation was performed. The chest cavity was opened and the hearts were isolated, placed in 0.9% solution of sodium chloride (physiological saline) to wash away the blood in the heart chambers and a transverse incision was performed through the middle of the heart, slightly below the level of the heart valves. The hearts were removed and post fixed in 4% solution of paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 overnight at 4ºC.
All tissues were fixed in 10% formalin solution for at least 24 h, dehydrated in alcohol, cleared in xylene and embedded in paraffin. Serial sections were cut on a microtome (Reichert-Jung) at a thickness of 7 µm. We used microscope slides processed with gelatin-coated glass as an adhesive. The obtained paraffin sections were then mounted on the microscope slides. Sections were stained routinely with hematoxylin and eosin (H&E) and Mallory’s trichrome stain; all according to standard methods.
Results
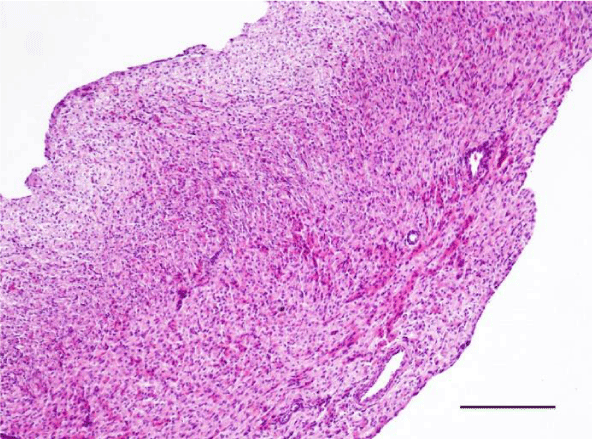
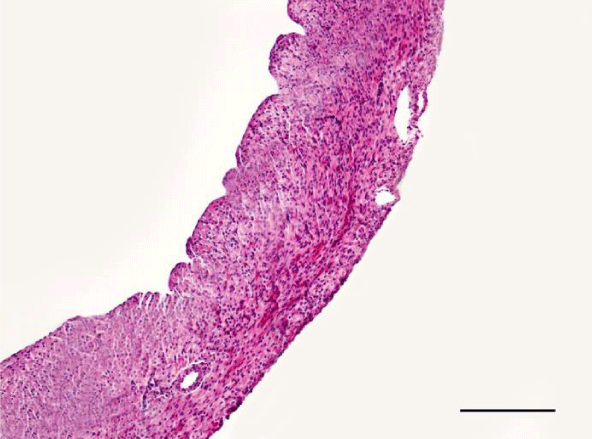
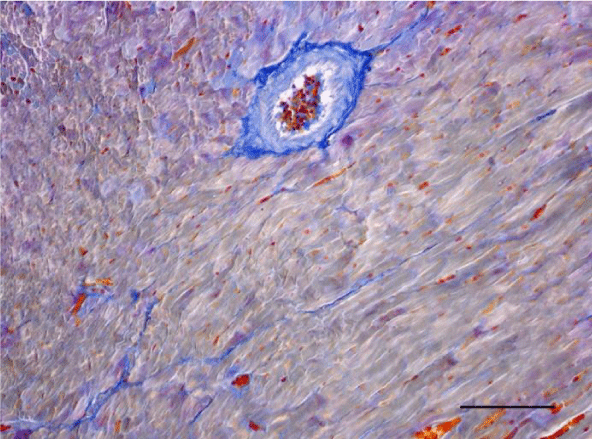
On H&E stained slides in 2-week-old rats, it was difficult to observe clearly defined longitudinal and transverse layers of cardiomyocytes in the wall of the left as well as the right ventricle. Cardiomyocytes appeared to be slightly eosinophilic and pronounced cross striation was not observed. They usually had one centrally located basophilic nucleus; in some cells, one or more nucleoli were noted. The perinuclear zone was well seen and was stained less intensively. The muscle fibers were enveloped by fairly thin connective tissue and a perimysium with abundant capillary network. It was fairly rare that fibroblastic nuclei were observed among the cardiac muscle cells (Figures 1,2).
Mallory’s trichrome stain in 2-week-old animals revealed a scarce amount of fine collagen fibers in the walls of the two ventricles. Very few thin and quite short connective tissue fibers were noted in the interstitial space of the left ventricle. More collagen fibers were situated predominantly in the perivascular zone and in the walls of the blood vessels and were stained in an intensive blue color; we noted a slight tendency for collagen fibers to grow from the perivascular zone towards the interstitial space (Figures 3,4).
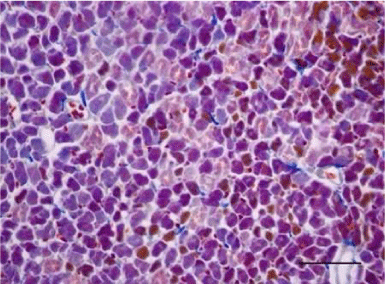
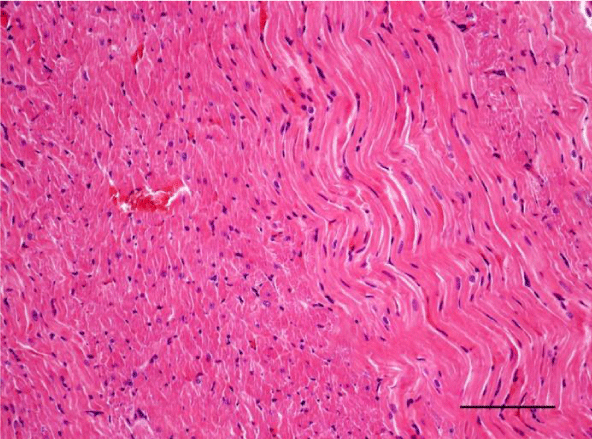
In the age groups of young rats (1- and 3-month-old), the H&E stain revealed a well-developed morphological organization of the layers of the myocardium in both ventricles. Bundles of longitudinally aligned cardiomyocytes were clearly observed, as well as subepicardial and subendocardial layers of transversely cut cardiac muscle cells. On a longitudinal section, cardiomyocytes from the left and right ventricle appeared eosinophilic, with cross striations and had one or two centrally located basophilic nuclei. The myofibrils appeared to be surrounding the nucleus and quite often, striations were noted in the perinuclear zone. The cardiomyocytes branched and formed anastomoses thus participating in a complex network. Each individual muscle fiber was enveloped by endomysium made up of fine connective tissue and perimysium with an abundant capillary network. Individual fibers were not always visualized but nuclei of fibroblasts and fibrocytes were identified among the cardiac muscle cells. The nuclei of the fibroblasts and fibrocytes were flat and stained more intensively than those of cardiomyocytes.
On a transverse section, the nuclei of the cardiomyocytes were centrally located and we observed that sometimes the perinuclear zone stained slightly more palely. Between the bundles of cardiac muscle cells we noted the presence of connective tissue, among which the nuclei of fibroblasts and fibrocytes were found. Some of them were located along the periphery of the muscle fiber due to each individual cardiomyocyte being enveloped by endomysium. Multiple capillaries were observed as small optically empty oval structures around the cardiomyocytes. The thickened areas of the capillary wall marked the location of the nuclei of the endothelial cells. Large intramural blood vessels were also seen, in the lumen of which we noted the presence of erythrocytes (Figures 5-8).
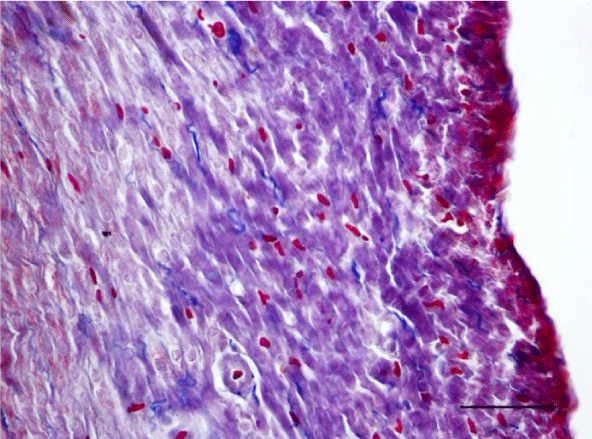
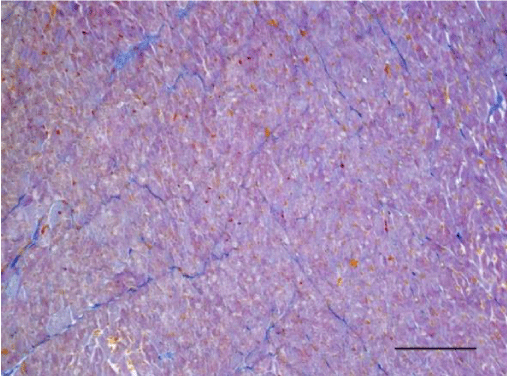
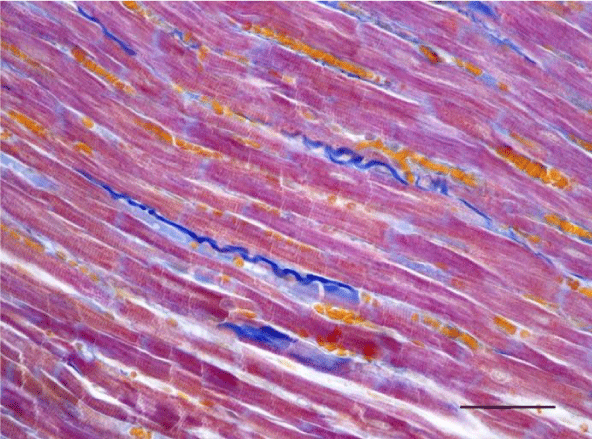
Mallory’s trichrome stain in young rats revealed an insignificant number of fine collagen fibers present predominantly in the perivascular zone and in the wall of the blood vessels in the left as well as the right ventricle (Figures 9,10). Collagen fibers were also visualized in the interstitial space and the perimysium, where their quantity was less and their alignment was not strictly organized. In the left ventricle of three-month-old animals they were fairly thicker and had a spiral shape unlike those in the left ventricle of one-month-old rats (Figure 11). They are relatively shorter and fewer in number. In the wall of the left ventricle within both age groups we noted extremely short and few collagen fibers located in the interstitial space (Figure 12). Thin and short fibrous fascicles were seen extending from the perivascular space among the cardiomyocytes in both ventricles.
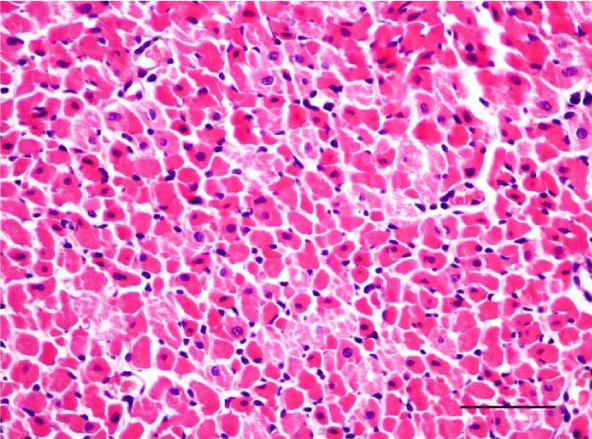
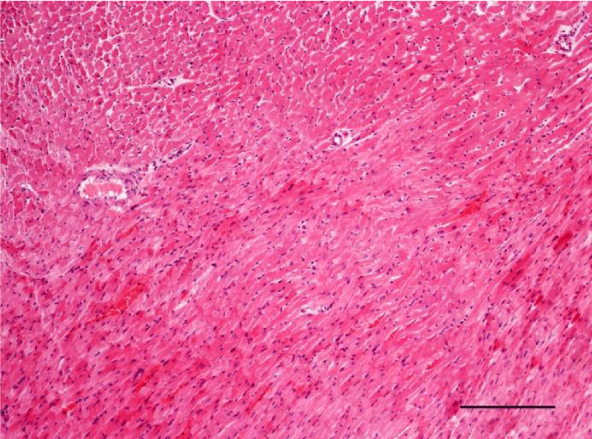
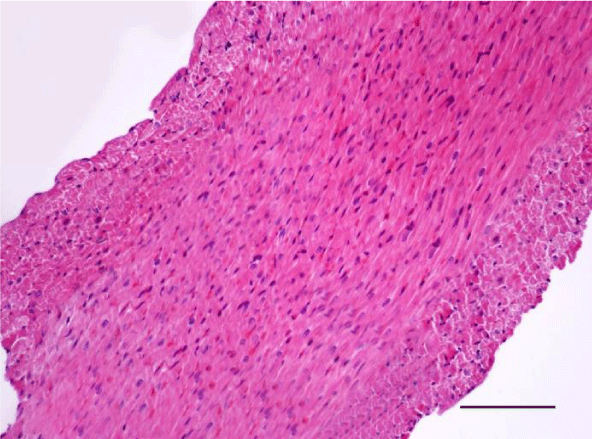
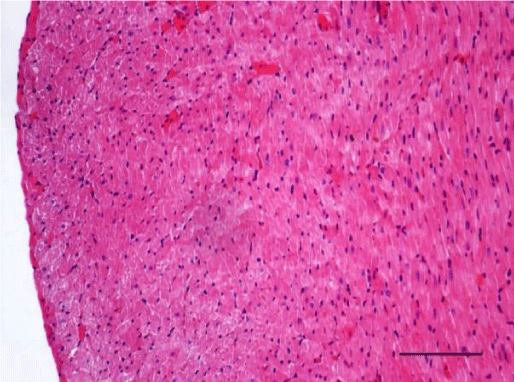
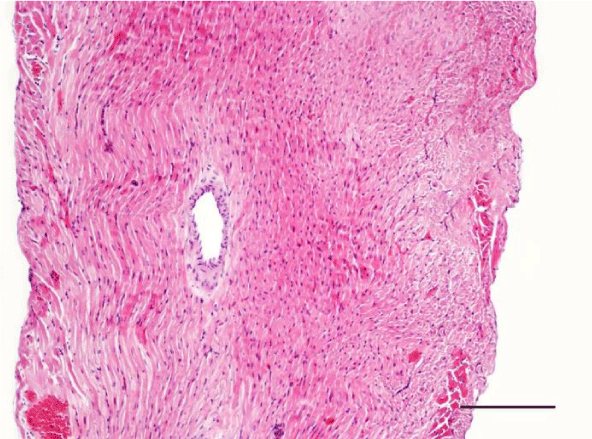
In adult six-month-old rats cardiomyocites from the left and right ventricle appeared eosinophilic on H&E staining, with cross striations and one or two centrally located basophilic nuclei. No striation was noted in the perinuclear zone. Well defined longitudinal and transverse bundles of cardiac muscle cells were seen. Each muscle fiber was enveloped by endomysium built of connective tissue and perimysium containing a capillary network. A certain number of fibroblastic and fibrocytic nuclei were observed among the cardiomyocytes. The presence of capillaries between the muscle cells was also noted. We reported the occurrence of cardiomyocytic hypertrophy and the narrowing of the interstitial space. A mild neutrophil infiltration and pyknotic nuclei were observed, as well as moderately pronounced subendocardial and interstitial fibrosis presenting as eosinophilic fascicles, extending among the cardiac muscle cells. Moderate hyaline degeneration, vacuolization and degradation of the sarcomeres were also noted (Figures 13,14).
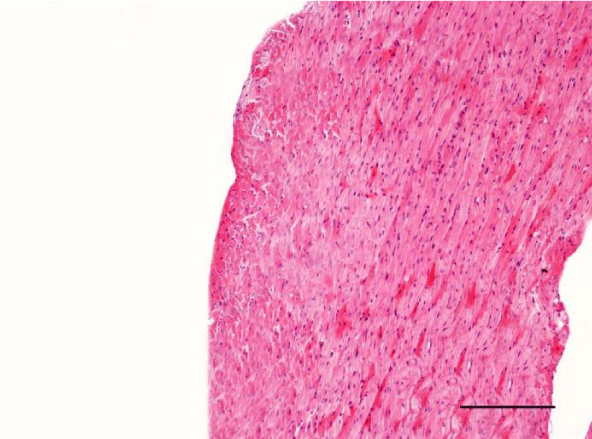
In the group of senescent twelve-month-old rats, age-related changes observed in the wall of the left and the right ventricle on H&E staining were best expressed. Bundles of large longitudinally positioned hypertrophic cardiac muscle cells with large basophilic nuclei with reticular structure were seen. We also noted bundles of hypertrophic cardiomyocytes from the transverse layers and further narrowing of the interstitial space. Capillaries in the connective tissue surrounding the muscle fibers were found less often. A well-pronounced neutrophil infiltration and the presence of pyknotic nuclei were observed. Subendocardial and interstitial fibrosis was more manifest and presented as clearly visible eosinophilic fascicles. A major finding was the focal myocytolysis: individual cardiomyocytes which appeared ‘empty’, i.e. no nuclei and cross striations were noted in them, while their cytoplasm stained more intensively eosinophilic (Figures 15,16). When comparing the two ventricles we noted that these changes in myocardial morphology were more extensive and advanced in the wall of the left as opposed to the right ventricle, where hypertrophic and necrotic cardiomyocytes were fewer in number.
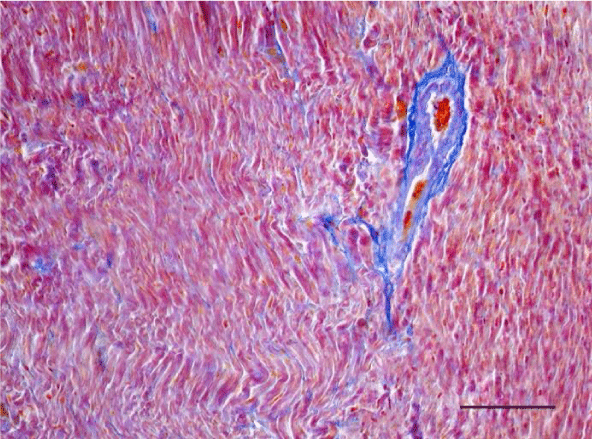
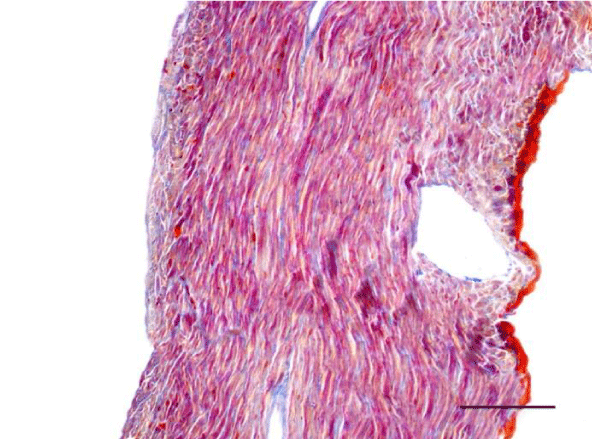
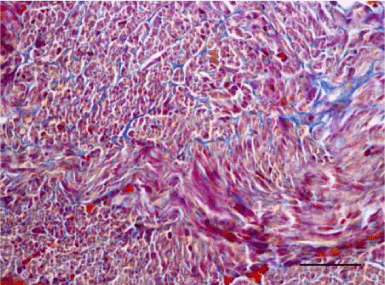
A moderate number of collagen fibers, predominantly in the perivascular zone, as well as the interstitial space, were reported on Mallory’s stain in the wall of the left ventricle in the group of adult (six-month-old) rats. We observed intensively blue-stained, fairly long collagen fibers in the interstitial space which formed complex anastomoses (Figure 17). In senescent (twelve-month-old) rats, collagen fibers in the wall of the left ventricle were abundant both in the perivascular zone and the interstitial space and their alignment was not strictly organized. They had a complex spiral shape and course and were much thicker. Connective tissue in the perimysium was more pronounced (Figure 18). Changes observed in the wall of the right ventricle of six- and twelve-month-old Wistar rats were identical to those described in the left ventricle, however they were characterized as moderate and less expressed. Collagen fibers were shorter and thinner. The perivascular connective tissue was less abundant and the extension of collagen fibers from the perivascular zone towards the interstitial space was less pronounced (Figures 19,20).
Discussion
On histological slides prepared from the hearts of the rats we traced the changes observed in different periods from the postnatal development of Wistar rats. Alterations in the morphology of the wall of the left and the right ventricle, as well as the changes in the cardiomyocytes were noted. It is a well-known fact that the most intensive period of body growth in rats is observed up to the second month after birth. For this reason, the condition of the myocardium throughout this period was traced in two age groups: two-week-old and one-month old animals. Furthermore, it has been documented that the most dynamic changes in the cardiomyocytes of the rat take place over the period before the onset of puberty (around 2 weeks after birth), as well as during the early post-puberty period (around 1 month after birth). After the second postnatal month growth rate decreases and the animals are considered adult in the sixth postnatal month. Therefore, analysis of the morphology of the cardiac wall in the left and right ventricle was also conducted in three- and six-month-old animals. According to literature data, life expectancy in rats is between 2 and 3.5 years [8]. After two years of age, the frequency of infectious and neoplastic diseases increases and a large portion of animals die. Therefore, the studied animals in the period of senescence in the present work were twelve months old. All rats selected for this study were in good overall condition and with no physically noticeable signs of disease.
In line with our results and literature data, aging of the myocardium is related to hypertrophy of the left ventricle and the development of fibrosis [9,10]. The gradual age-related deposition of collagen in the walls of the vessels, the interstitial and perivascular space, demonstrated in the present study in different age groups of Wistar rats, confirms the results of the studies of Lakatta and Levy (2003) [9]. The increased development of fibrosis is a fundamental prerequisite for myocardial rigidity which, together with the impaired relaxation of the ventricles, leads to myocardial dysfunction [11]. Over the course of development of fibrotic changes, an increased tendency for deposition of collagen in the peri- and endomysium is noted, which leads to interstitial fibrosis, while the deposition of collagen in the adventitia of intramural coronary vessels leads to perivascular fibrosis [12]. These data are supported by the present study.
In healthy Wistar rats, aging is related to changes in myocardial morphology. Age-related remodeling of the cardiac wall is related to cardiomyocytic hypertrophy and interstitial fibrosis. In physiological conditions, the bundles of cardiomyocytes and the individual cells are enveloped by thin layers known as peri- and endomysium. In the aging myocardium, transformation of the fibroblasts into myofibroblasts and accumulation of extracellular matrix proteins in the interstitial space are observed. Histological analyses of aging hearts from Wistar rats show progressive loss of cardiomyocytes due to necrosis and apoptosis [13]. Preserved cardiac muscle cells undergo a process of compensatory hypertrophy [14]. Collagen content increases as aging progresses, therefore collagen fibers in the endo- and perimysium of older animals are thicker [15]. This was well noted in the present study, where a tendency for collagen fibers to become thicker and to acquire a complex spiral shape was observed as aging progressed in both ventricles but was significantly more pronounced in the myocardium of the left ventricle, which was attributed to the higher afterload exerted on the left ventricle and consequently, the higher degree of injury suffered by the ventricle with progression to senescence.
The loss of cardiomyocytes due to necrosis and apoptosis leads to compensatory or reactive hypertrophy of the remaining cardiomyocytes [16]. Cardiomyocytic hypertrophy itself is a physiological process taking place in response to increased demands (such as physical exercise) and therefore, age-related hypertrophy can be considered as developing through a similar mechanism. Unlike physiological hypertrophy, age-related hypertrophy is a result of loss of cardiomyocytes due to aging and the decrease in their stability, which increases workload on the remaining healthy cells [17]. This hypothesis was confirmed by the results of our study. We observed initial hypetrophic changes in the group of adult Wistar rats, which progressed to well-developed hypertrophy in the group of senescent animals and combined with the presence of focal myocytolysis, which is the histological equivalent of cell necrosis. These morphological changes were better expressed and had a more dynamic course in the wall of the left ventricle. This led us to the hypothesis that the relative preservation of the normal morphology of the right ventricle may be associated with a higher reserve capacity to respond to increased stress. It can be stated that the processes of accumulation of interstitial collagen and cardiomyocytic hypertrophy with the onset of senescence show that the morphology and function of the heart becomes impaired not only at the level of the organ but at the level of the individual cardiomyocyte as well [17].
Literature data make it evident that in senescent Wistar rats, the formation of fibrosis in the right ventricle increases with age [17]. One study found development of fibrosis and accumulation of adipose tissue in the cardiac conduction system [18]. A study of Klima et al. [19], demonstrated a significant development of fibrosis, especially in the wall of the left ventricle. Our data from the morphological analysis of Mallory’s trichrome stain in all age groups of rats show an increase in the collagen content as aging progressed in both ventricles. In the group of senescent Wistar rats we noted a significant difference between the intensity of staining between histological slides from the left and the right ventricle, with the left ventricle staining more intensively blue with notable visualization of the collagen fibers which corresponded to a more pronounced accumulation of collagen.
It is considered that cardiomyocytic hypertrophy results from the impairment of the balance between the signal pathways of hypertrophy and atrophy [20]. The low rate of cellular death in the normal heart, as well as the presence of cardiomyocytic hypertrophy and fibrosis as mechanisms intended to compensate cellular loss probably decrease the need for cardiomyocytic regeneration [14]. New data present the hypothesis of a probable existence of two sources of cardiomyocytic renewal: division of already existing cardiomyocytes and differentiation of precursor cells [21]. The loss of cardiomyocytes as aging progresses probably results from two separate mechanisms: loss of cells due to depletion of adaptive mechanisms (impaired division of cardiomyocytes and/or of the maturation of the cardiac progenitor cells) and active loss of cells due to activation of the process of apoptosis [22]. All of this leads to impairment of the myocardial function and is therefore a key process in the overall incidence of pathological conditions such as heart failure and cardiac rhythm and conduction disorders.
Conclusion
In conclusion, aging of the myocardium is a dynamic process characterized by cardiomyocytic hypertrophy and increased deposition of collagen in the interstitial space. Considering the functional specifics of the work of the left and right ventricle (namely a more pronounced effect of afterload on the left ventricle), processes in the left ventricle follow a more dynamic course and reactive fibrosis occurs earlier. These processes advance relatively smoothly in the right ventricle.
- Marian AJ, Roberts R (2001) The molecular genetic basis for hypertrophic cardiomyopathy. J Mol Cell Cardiol 33: 655-670. Link: https://goo.gl/3pF6oz
- Kostin S, Scholz D, Shimada T, Maeno Y, Mollnau H, et al. (1998) The internal and external protein scaffold of the T-tubular system in cardiomyocytes. Cell Tissue Res 294: 449-460. Link: https://goo.gl/RWn6Bf
- Legato MJ (1973) Ultrastructure of the Atrial, Ventricular, and Purkinje Cell with Special Reference to the Genesis of Arrhythmias. Circ 47: 178-189. Link: https://goo.gl/weUVuj
- Gupta M, Gupta MP (1997) Cardiac hypertrophy: old concepts, new perspectives. Mol Cell Biochem 176: 273-279. Link: https://goo.gl/50Rn0O
- Kumarapeli AR, Wang X (2004) Genetic modification of the heart: chaperones and the cytoskeleton. J Mol Cell Cardiol 37: 1097-1109. Link: https://goo.gl/hO9Ycy
- Miller MK, Granzier H, Ehler E, Gregorio CC (2004) The sensitive giant: the role of titin-based stretch sensing complexes in the heart. Trends Cell Biol 14: 119-126. Link: https://goo.gl/OYbBpc
- Perriard JC, Hirschy A, Ehler E (2003) Dilated cardiomyopathy: a disease of the intercalated disc? Trends Cardiovasc Med 13: 30-38. Link: https://goo.gl/wxIiPC
- Sharp PE, La Regina MC (1998) The laboratory rat. CRC Press Boca Raton. Link: https://goo.gl/GzoV8m
- Lakatta EG, Levy D (2003) Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation 107: 346-354. Link: https://goo.gl/ZlvA3y
- Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, et al. (2001) Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol 87: 413-419. Link: https://goo.gl/Ppak3k
- Burlew BS (2004) Diastolic dysfunction in the elderly--the interstitial issue. Am J Geriatr Cardiol 13: 29-38. Link: https://goo.gl/JWZ0fZ
- Biernacka A, Frangogiannis NG (2011) Aging and Cardiac Fibrosis. Aging Dis 2: 158-173. Link: https://goo.gl/arbC12
- Kajstura J, Cheng W, Sarangarajan R, Li P, Li B, et al. (1996) Necrotic and apoptotic myocyte cell death in the aging heart of Fischer 344 rats. Am J Physiol 271: H1215-H1228. Link: https://goo.gl/oKhlu0
- Anversa P, Palackal T, Sonnenblick EH, Olivetti G, Meggs LG, et al. (1990) Myocyte cell loss and myocyte cellular hyperplasia in the hypertrophied aging rat heart. Circ Res 67: 871-885. Link: https://goo.gl/cDEdoR
- Chimenti C, Kajstura J, Torella D, Urbanek K, Heleniak H, et al. (2003) Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ Res 93: 604–613. Link: https://goo.gl/e8YWWj
- Bernhard D, Laufer G (2008) The aging cardiomyocyte: a mini-review. Gerontology 54: 24-31. Link: https://goo.gl/HdhfWG
- Burkauskiene A, Mackiewicz Z, Virtanen I, Konttinen YT (2006) Agerelated changes in myocardial nerve and collagen networks of the auricle of the right atrium. Acta Cardiol 61: 513-518. Link: https://goo.gl/kFV4ad
- Song Y, Yao Q, Zhu J, Luo B, Liang S (1999) Age-related variation in the interstitial tissues of the cardiac conduction system; and autopsy study of 230 Han Chinese. Forensic Sci Int 104: 133-142. Link: https://goo.gl/hQcCs6
- Klima M, Burns TR, Chopra A (1990) Myocardial fibrosis in the elderly. Arch Pathol Lab Med 114: 938-942. Link: https://goo.gl/xhlsq7
- Razeghi P, Taegtmeyer H (2006) Hypertrophy and atrophy of the heart. Ann Nw Y Acad. Sci 1080: 110-119. Link: https://goo.gl/L5LyVJ
- Nadal-Ginard B, Kajstura J, Leri A, Anversa P (2003) Myocyte death, growth, and regeneration in cardiac hypertrophy and failure. Circ Res 92: 139-150. Link: https://goo.gl/NYX65C
- Shih H, Lee B, Lee RJ, Boyle AJ (2011) The aging heart and post-infarction left ventricular remodeling. J Am Coll Cardiol 57: 9-17. Link: https://goo.gl/fWDA3e
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.




 Save to Mendeley
Save to Mendeley
