Archives of Anatomy and Physiology
Circadian Variations of Serum Mitochondrial Uncoupling Protein 1 (UCP1) Levels and Rectal Temperature in Capra Hircus
Francesca Arfuso, Claudia Giannetto, Francesco Fazio, Maria Rizzo, Concetta Saoca and Giuseppe Piccione*
Cite this as
Arfuso F, Giannetto C, Fazio F, Rizzo M, Saoca C, et al. (2017) Circadian Variations of Serum Mitochondrial Uncoupling Protein 1 (UCP1) Levels and Rectal Temperature in Capra Hircus. Arch Anat Physiol 2(1): 007-010. DOI: 10.17352/aap.000004Background: The daily rhythm of body temperature is an important process to be studied not only to advance knowledge on the temporal variability of thermal homeostasis but also has a means to facilitate the study of biological rhythmicity in general. The aim of the present study was to study the daily rhythm of rectal temperature and the changes in the serum levels of mitochondrial uncoupling protein 1 (UCP1) in goat.
Methods: On five female goats rectal temperature was recorded at 4 hour intervals for a 24 hour period. At the same time points blood samples were collected from each animal.
Results: Daily rhythm of rectal temperature was observed in studied goats, whereas UCP1 did not show daily rhythmicity.
Conclusion: The results obtained in the present study suggest that the regulation of rectal temperature is primarily under the circadian control. The absence of rhythmicity of UCP1serum levels suggest a lack of synchronization of this protein in the blood and that, probably, the activity of this protein is auxiliary to keeping the body temperature daily rhythm.
Abbreviations
SH: Shivering Thermogenesis; NST: Non-Shivering Thermogenesis; BAT: Brown Adipose Tissue; UCP1: Uncoupling Protein 1.
Introduction
In homoeothermic organisms, the core body temperature is regulated within a narrow range by a complex feedback system. The thermoregulatory center controls mechanisms aimed at equalizing heat production and heat lose. Two strategies are used for heat production: shivering thermogenesis (SH), involving skeletal muscles, and non-shivering thermogenesis (NST). A major site of mammalian NST is brown adipose tissue (BAT), characterized by high glucose uptake, oxidative capacity and mitochondrial uncoupling [1]. Thermogenesis in BAT results from an increase in the rate of substrate oxidation in mitochondria caused by a proton conductance pathway through the uncoupling protein 1 (UCP1) [2].
Thermal physiologists have generally assumed that the circadian rhythm of body temperature is primarily under homeostatic control and it is secondarily modulated by the circadian system through an oscillation in the thermoregulatory set point [3,4].
From the viewpoint of the circadian physiologist, it is more sensible to assume that the circadian oscillation in body temperature is primarily under circadian control, bypassing the thermoregulatory set point, and is secondarily modulated by the thermoregulatory system [5]. Circadian rhythm of body temperature has been found in many mammals’ species [6]. It has been suggest that BAT is neuroanatomical connected with the SCN, indicating the possible involvement of BAT in associations between disturbed circadian rhythm and obesity [7]. In human, it has been suggested that BAT activity is also physiologically regulated by the biological clock [7]. The presence of BAT in adult mammals is a recent discovery, thus conflicting results on the UCP1 presence and function in adult animals are found in the literature [8-11].
Despite a substantial literature about the physiological aspects of body temperature maintenance in goat species, few studies have been conducted to investigate the mechanisms controlling the circadian thermogenic rhythm, whereas no information on daily rhythm of serum UCP1 levels is available in the current literature. The aim of the present study was to study the daily rhythm of rectal temperatures and serum UCP1 values in goats in order to improve the knowledge about the primary role of circadian or thermoregulatory system on the homeostasis of body temperature.
Materials and Methods
Five not pregnant and not lactating female Maltese goats aged 3-4 year old and with a mean body weight of 44±3 kg were enrolled in the study. All the animals enrolled in the study were clinically healthy with no evidence of disease and free from internal and external parasites. Their health status was evaluated based on heart rate, respiratory rate, appetite, faecal consistency and hematologic profile. Fresh fecal samples were examined according to the MC MASTER Method based on protocols previously described by Maffa [12].
The animals were individually housed in a 3.5x3.5 meters box equipped with an opening window under natural photoperiod (sunrise 05:05, sunset 20:55) and environmental temperature and humidity (24-28°C, 62%). Thermo-hygrometric recordings were conducted inside the stable throughout the entire study by means of a data logger (Gemini, Chichester, West Sussex, UK).
The goats had free access to good-quality hay and to water. General animal care was carried out by professional staff not associated with the research team. Protocols of animal husbandry and experimentation were reviewed and approved in accordance with the standards recommended by the Guide for the Care and Use of Laboratory Animals and Directive 86/609CEE.
Data collection was performed every 4 hours over a 24 hour period. Dim red light (< 3 lux, 15 W Safelight lamp filter 1A, Kodak Spa) was used for data collection during the dark phase of the natural L/D cycle. Rectal temperature was recorded with a digital thermometer (HI92704, Hanna Instruments Bedfordshire, UK), with resolution of 0.1ºC, that was inserted 9 cm into the rectum. Blood samples were collected by means of jugular venipuncture using vacutainer tubes with no additive (Terumo, Japan). Blood samples were allowed to clot for two hours at room temperature, thereafter, they were centrifuged at 1000 x g for 20min and the obtained sera were stored at -20°C until analysis. Only the not hemolysed obtained sera were analysed to evaluate the concentration of mitochondrial uncoupling protein 1 (UCP1) using ELISA kit (Goat Uncoupling Protein 1, Mitochondrial (UCP1) ELISA Kit, MyBioSource, Inc. San Diego, California, USA) by means of a microtiter plate reader (EZ Read 400 ELISA, Biochrom, Cambridge, United Kingdom). All calibrators and samples were run in duplicate and samples exhibited parallel displacement to the standard curve for the ELISA analysis. Both the intra- and the inter-assay coefficients of variation for UCP1 were of < 15%.
All the results were expressed as mean ± standard deviation (SD). Data were normally distributed (P > 0.05, Kolmogorov-Smirnov test). We applied a trigonometric statistical model to each subject values of each time series, so as to describe the periodic phenomenon analytically, by characterizing the main rhythmic parameters according to the single cosinor procedure [13]. Four rhythmic parameters were determined: mesor, amplitude (the difference between the peak, or trough, and the mean value of a wave), acrophase (the time at which the peak of a rhythm occurs), and robustness (strength of rhythmicity).
ResultsResults
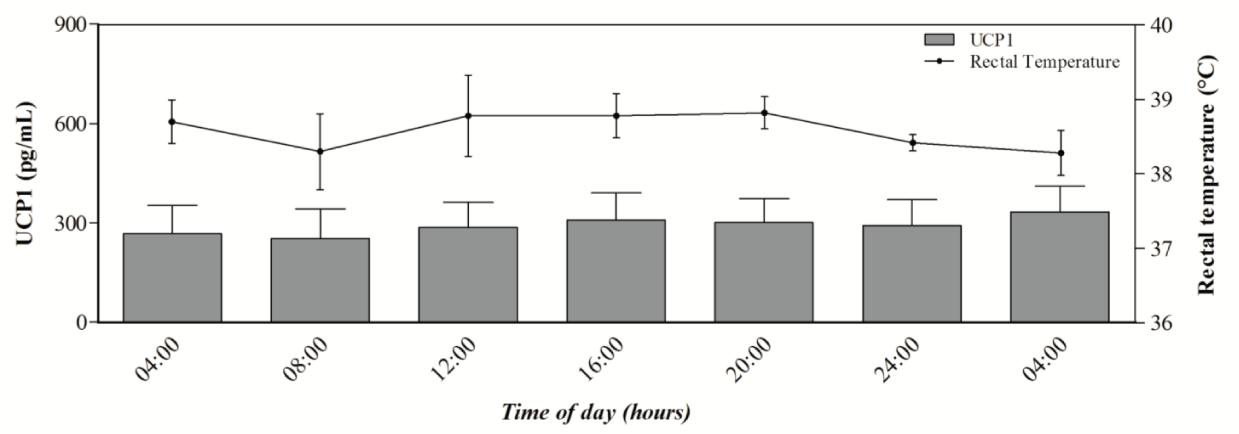
The single cosinor method showed a daily rhythm of rectal temperature in studied goats during the day of monitoring (P < 0.05), whereas UCP1 oscillation did not show daily rhythmicity (Table 1).
Figure 1 shows the rhythmic trend of oscillation of rectal temperature and the mean values of serum UCP1 recorded during the experimental period in goats.
Discussion
The evaluation of body temperature represents a valuable tool to monitor the physiologic status, welfare and the stress responses of animals. The regulation of body temperature in homeotherms is ensured by mechanisms of thermolysis and thermogenesis. Thermoregulatory adjustments can be induced not only by changes in environmental temperature but also by a variety of physiological situations [14]. The results found in this study showed a daily rhythm of body temperature in goats. The circadian changes in core temperature are probably due to a rhythmic input from the suprachiasmatic nuclei (SCN) acting upon the hypothalamic thermoregulatory system, modulating the set point and altering the thresholds for cutaneous vasodilatation and sweating [15,16]. Rectal temperature may sometimes exceed core temperature or lag significantly from these other body sites [17].
It is stated that the circadian rhythm of body temperature is determined both by circadian changes in heat production and heat loss, with the rhythm of heat production being phase-advanced with respect to the rhythm of heat loss [18]. Therefore, daily fluctuation of body temperature is the results of a continuous interplay between circadian and homeostatic mechanism. The circadian rhythms generated by master clock neurons of SCN and its efferent targets in the hypothalamus integrate environmental signals to entrain behavioral rhythms as well as clock cells located in peripheral tissues including liver, muscle and adipose tissue. Several authors [7] established that BAT expressed intrinsic circadian rhythms of specific genes involved in lipid biosynthesis, fatty acid oxidation and adaptive thermogenesis suggesting a link between circadian and thermogenic networks. The adaptive thermogenesis is defined as heat production in response to environmental temperature and serves the purpose of protecting the organism from cold involving stimulation of lipolysis and increase in activity and production of UCP1. The UCP1 is enriched in BAT that is the principal site of thermoregulatory heat production in the young of many mammalian species.
Recently a new typology of adipocytes, known as brown-in-white, or beige adipocytes, has been identified in adult animals. These adipocytes show an expression panel partially typical of white adipocytes, and show a role in thermogenesis vie expression of UCP1, as well [19]. Beige adipocytes have a very low basal level of UCP1, however, the production of this uncoupling protein increased considerably after cold exposure or different stimulation [19]. It has been reported that UCP1 production is coordinated by the clock gene Per2 [20]. This seems to suggest that UCP1 levels follow a circadian rhythm in adipose tissue. However, it is unknown whether serum values of UCP1 maintain a rhythmic trend. The results obtained in the present study showed that the serum values of UCP1 did not show daily rhythmicity in goats suggesting a lack of synchronization of this protein at serum level where its rhythm may have been abolished.
Conclusion
The results obtained in the present study confirm that the body temperature is under the circadian control in goat species, whereas the serum levels of UCP1 is not rhythmic in adult goats, and probably the activity of this protein is auxiliary to keeping the body temperature daily rhythm.
- Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277-359. Link: https://goo.gl/wFqUmj
- Rousset S, Alves-Guerra MC, Mozo J, Miroux B, Cassard-Doulcier AM, et al. (2004) The biology of mitochondrial uncoupling proteins. Diabetes 53: S130-S135. Link: https://goo.gl/OpaCnn
- Refinetti R (2010) The circadian rhythm of body temperature. Front Biosci 15: 564-594. Link: https://goo.gl/PHsN6s
- Piccione G, Bertolucci C, Costa A, Di Mauro S, Caola G (2006) Daily rhythm of body and auricle temperature in goats kept at two different ambient temperatures. Biol Rhythm Res 36: 309-314. Link: https://goo.gl/z0qOTo
- Refinetti R (2006) Circadian physiology. 2nd edition, Taylor and Francis, Boca Raton. Link: https://goo.gl/oaj1Nr
- Piccione G, Refinetti R (2003) Thermal chronobiology of domestic animals. Front Biosci 8: S258-S264. Link: https://goo.gl/3n65wF
- Kooijiman S, van den Heuvel JK, Rensen PCN (2015) Neuronal control of brown fat activity. Trends Endocrinol Metab 26: 657-668. Link: https://goo.gl/UGDF0c
- Arfuso F, Giannetto C, Rizzo M, Fazio F, Giudice E, et al. (2016) Serum levels of mitochondrial uncoupling protein 1, leptin, and lipids during late pregnancy and the early postpartum period in mares. Theriogenology 86: 1156-1164. Link: https://goo.gl/MDbUqT
- Arfuso F, Rizzo M, Giannetto C, Giudice E, Fazio F, et al. (2016) Age related changes of serum mitochondrial uncoupling 1, rumen and rectal temperature in goats. J Thermal Biol 59: 47-51. Link: https://goo.gl/AeVBxS
- Asano H, Yamada T, Hashimoto O, Umemoto T, Sato R, et al. (2013) Diet-induced changes in UCP1 expression in bovine adipose tissues. Gen Comp Endocrinol 184: 87-92. Link: https://goo.gl/VCTrSa
- Henry BA, Blache D, Rao A, Clarke IJ, Maloney SK (2010) Disparate effects of feeding on core body and adipose tissue temperatures in animals selectively bred for nervous or calm temperament. Am J Physiol Reg Integr Comp Physiol 299: R907-R917. Link: https://goo.gl/WKqveA
- Maffa (1989) MAFF, Ministery of Agriculture Fisheries and Food - Manual of veterinary laboratory diagnostic techniques. Reference Book No. 418. Her Majesty's Stationery Office, London. Link: https://goo.gl/xFIEg3
- Nelson W, Tong YL, Lee JK, Halberg F (1979) Methods for cosinor rhythmometry. Chronobiol 6: 305-323. Link: https://goo.gl/2t1o9N
- Weinert D (2010) Circadian temperature variation and ageing. Ageing Res Rew 9: 51-60. Link: https://goo.gl/4YK2OT
- Refinetti R, Menaker M (1992) The circadian rhythm of body temperature. Physiol Behav 51: 613-637. Link: https://goo.gl/r1gltm
- Refinetti R (1997) Homeostasis and circadian rhythmicity in the control of body temperature. Ann NY Acad Sci 813: 63-70. Link: https://goo.gl/V8plrR
- Fraden J, Lackey RP (1991) Estimation of body site temperatures from tympanic measurements. Clin Pediatr 30: 65-70. Link: https://goo.gl/WXkPWz
- Aschoff J, Heiss A (1972) Thermal conductance in man: its dependence on time of day and on ambient temperature. In: Itoh A, Ogata K, Yoshimura H. (Eds.), Advances in climatic physiology. Igaku Shoin, Tokyo, 1334-1364. Link: https://goo.gl/7rN34K
- Wu J, Boström P, Sparks LM, Ye L, Choi JH, et al. (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150: 366-376. Link: https://goo.gl/lKrHQV
- Chappuis S, Ripperger JA, Schnell A, Rando G, Jud C, et al. (2013) Role of the circadian clock gene Per2 in adaptation to cold temperature. Mol Metab 2: 184-193 Link: https://goo.gl/ZYAjmN
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
