Annals of Antivirals and Antiretrovirals
A comprehensive overview of the pharmaceutical properties of Indian coastal sand dune flora: Emphasis on anti-virals
Saravanakumar Vigneshwar1, Arjun Kowsalya2, Sarah Jency John Kennedy3, Praveen Gopi3 and Dinakarkumar Yuvaraj3*
2Anna University Tiruchirappalli, BIT CAMPUS, Tiruchirappalli, Tamil Nadu, 620024, India
3Department of Biotechnology, Vel Tech High Tech Dr. Rangarajan Dr. Sakunthala Engineering College, Chennai, Tamil Nadu, 600042, India
Cite this as
Vigneshwar S, Kowsalya A, John Kennedy SJ, Gopi P, Yuvaraj D (2023) A comprehensive overview of the pharmaceutical properties of Indian coastal sand dune flora: Emphasis on anti-virals. Ann Antivir Antiretrovir 7(1): 001-009. DOI: 10.17352/aaa.000016Copyright
© 2023 Vigneshwar S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Viral infections have an essential function in both humans and other organisms. The persistent rise in viral diseases has made critical damage to human well-being. The present review indicates that viral diseases are been entangled in various cancer developments. Developing safe and effective antiviral medications remains a challenge. As a result, finding therapeutic targets that would interfere with the virus without affecting the host is hard-hitting. The use of natural substances rather than chemicals in the formulation of antiviral medications could significantly minimize the risk of side effects in patients. Coastal dune vegetation is a vital resource, which plays an imperative part in biodiversity. Coastal dunes have various utilizations in restorative and drug development. The drugs from marine are vitally been utilized as medicine due to their substantial antiviral, anticancer, and antimicrobial activities. Though Coastal dunes flora has numerous possessions their antiviral properties are rarely reported. Hence, in this report, we have compiled and highlighted the antiviral properties of 128 Indian coastal dune flora. This review may provide access to a profound understanding of coastal dunes’ vegetation resources and their usage in the production of antiviral and anticancer drugs. It may also help to preserve and cultivate these plants.
Introduction
The persistent emergence of some developing or reappearing viral diseases has made unadulterated damage to human well-being. The unique structure of the viral infections and their muddled life cycle made it extremely difficult to explore conclusive therapies against viral diseases. Viral infections are extremely minute toxins that are made of hereditary material within a protein covering, that destroys healthy cells. This can injure, harm, or alter the cells and cause illness to people [1]. Distinctive viral infections can influence numerous zones in the body, including the conceptive, skin, cerebrum, blood, breathing system, liver, respiratory, and gastrointestinal systems [2]. For most popular viral diseases, medicines can just assist with manifestations while the immune system will battle off the viral infection. Antibiotic agents are not significant for viral diseases.
Several studies expose that viral infection is involved in numerous malignant growths such as cancer and tumor [2]. Viral infections cause regular colds, moles, and flu. They likewise cause extreme diseases including Influenza, Dengue, Zika virus, Herpes Simplex Virus (HSV), Chikungunya, Hepatitis A (HSV), Hepatitis B (HSB), Hepatitis C (HCV), AIDS, Ebola, chickenpox, Human Immunodeficiency Infection (HIV) and COVID-19 [1]. Antiviral medications are available for treating some popular viral diseases. Immunizations and vaccines can assist with keeping from getting numerous viral illnesses.
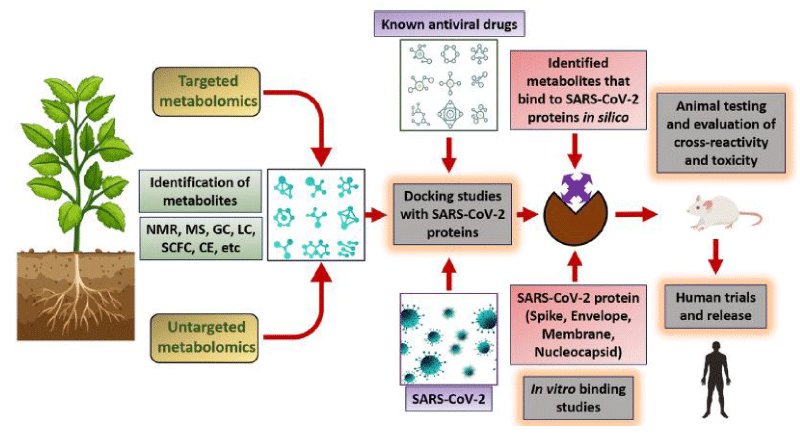
Due to the recent SARS-CoV-2 pandemic, the entire world is going through a very difficult time. To address this dire situation, therapeutic agents and vaccines against this virus are urgently needed. National Institute of Plant Genome Research, New Delhi, India, helps with research readiness in the fight against this virus. Three research groups are working hard to develop plant-based vaccines against SARS-CoV-2 and to investigate plant natural products that may be anti-viral in nature. The potential anti-SARS-CoV-2 activity of the identified molecule/s is being investigated in collaboration with the International Centre for Genetic Engineering and Biotechnology in New Delhi and the Regional Centre for Biotechnology in Faridabad, India (National Institute of Plant Genome Research (NIPGR), New Delhi, 2020) [3]. Marine plants and animals have a wide range of natural compounds, which are essentially novel, conceptually revolutionary, and have pharmacological effects [1]. To substantiate the above, this study focuses on the popular anti-viral properties of vegetation on coastal sand dunes Figure 1.
Coastal sand dunes vegetation
Coastal sand dunes are naturally dynamic. A coastal sand dune is a slope of sand effort by wind activity and an expansion of the sea shore into the land. While a seashore is firmly connected to the ocean and constrained by waves and tides, the ridges are connected to the land and are constrained by winds [4]. Due to interoperability issues, mobility, substrate versatility, and physical cycles, the coastal sand dunes comprise a variety of microenvironments [1].
Coastal sand dunes serve as a home for certain essential plants and animals (rare and endangered species) [4]. Coastal flora and vegetation are related to resilience to the consistency and saltiness slope of residue, wind, marine vaporization, and the nearness of bitter water [5]. Coastal sand dunes provide fundamental biological system management as environments for local and imperiled species, a site that gives high travel industry esteem, groundwater revives zones, and properties from wave disintegration and tempest flooding [6]. Coastal sand dunes are substantially involved in various vegetation, fauna, and microorganisms [6].
Applications of coastal sand dunes vegetations
The sand dune flora is an extremely essential resource in the healing, therapeutic, and economic [7]. The medications acquired from the ocean have the chance of exploiting in medication since it has enormous antiviral, antimicrobial, and anticancer activities [8]. The applications of coastal sand dunes include nutrients, feed, manure, flooder, nourishment, drug, firm and social uses [7]. Coastal sand dunes have been accounted for to contain a greater assortment of horticultural, agronomical, industrial, pharmaceutical, and chemically significant microorganisms [6].
All the clinical plants of coastal sand dunes were administered intravenously with added substances, for example, oil (coconut, sesame, and castor), milk and milk merchandise (buttermilk and ghee), normal salt, jaggery and nectar or applied remotely as a mixture, decoction, glue, or powder [8]. The greater parts of the plants utilized in medications are either blended in with different fixings or single. Many of these species varieties are known to be utilized in different medicines, as for relieving Jaundice, headache cure, dental abscess, hepatitis, mumps, dermatitis, cut, sinus headache, recuperating wounds, throat contamination, loose bowels, tingles, skin maladies, fix migraine, stomach ulcer, tumor, ear-hurt, eye torment, diabetes, colds, asthma, chest infections, and pneumonia in general [8].
Coastal sand dunes have numerous uses in the medicinal and pharmaceutical industries. Coastal sand dunes vegetations have an assortment of applications in the field of medication and drug ventures, for instance, vegetations of coastal sand dunes can be utilized as moderating, hypocholesterolemic, anti-acne, harm preventive, antihepatotoxicity, nematicide, antihistaminic, against eczematic, against skin break out, antiarthritic, mitigating, threatening to coronary, antiandrogenic, flavor, hemolytic, spermatogenic, hypocholesterolemic, slightness genic insectifuge, anti-inflammatory, hostile to coronary, immunostimulant, chemo-preventive, pesticide, torment easing, hostile to diabetic, pain relieving, cell support, against dermatitic, antileukemic, antitumor, anticancer, hepatoprotective, antispasmodic, antiasthma, diuretic, 5-alpha reductase inhibitor [9]. Coastal sand dunes have numerous properties or capacities, in spite of the fact that their antiviral properties are seldom referenced.
Antiviral activity and other therapeutic properties of coastal sand dunes vegetation (Table 1)
Challenges and future prospects: There is widespread agreement that plant metabolites have the potential to be novel antiviral agents against many viral diseases. These vegetation are abundant in coastal regions, have long been used in traditional medicine, and are thus prime candidates for the discovery of new bioactive metabolites. Several studies have been conducted to establish a link between the empirical uses of these plants to treat infections and photochemistry evidence of the compounds that underpin antiviral effects. However, efforts to investigate new pharmaceutical compounds and demonstrate their effect in vitro have not yet resulted in an antibiotic that is clinically beneficial and economically profitable. Concerns about drug-resistant microbes have heightened interest in plant-derived, effective antimicrobial compounds. Plants that have been used to treat infectious diseases for centuries now have a new lease on life. The detection and quantification of known, and even the discovery of new, small bioactive molecules produced by plants as secondary metabolites will open up new avenues of treatment for a wide range of infectious and noninfectious diseases. Extraction methods that are appropriate and optimised, susceptibility tests, and clinical trials are still required. The prospects for future research appear promising, with the potential discovery of new and effective treatments leading to significant advances [88,89]. Plant metabolomics based on mass spectrometry is an extremely powerful approach that is likely to provide comprehensive metabolite profiles of medicinal halophytes in the near future. In vitro tests are the first step in screening promising metabolites for antimicrobial effects, whether purified or in mixtures. Because of the complexity and diversity of chemical properties of plant metabolites, a combination of analytical platforms is required to increase the detection coverage of these compounds in biological samples. To detect volatile and nonvolatile metabolites, GC and HPLC coupled with mass spectrometry, as well as other techniques such as UPLC or NMR, are required to ensure that all or the majority of compounds are separated, detected, identified, quantified, and characterised. Each method should also cover a variety of extraction solvents in order to detect both polar and nonpolar compounds. The isolation and chemical characterization of each compound using NMR technologies, as well as testing them in bioassays, are critical steps toward determining each compound’s biological activity. Methodological approaches for in vitro tests, on the other hand, must be carefully tailored to the chemical nature of the metabolites or extracts. Protocols must be standardised and validated against representative biological models in order for product comparisons to be reliable and meaningful. Sand dune species serve as extremely important reservoirs. However, these resources have been put at greater risk as a result of forest clearing for industrialization, rapid urbanisation, over-exploitation and anthropogenic activities. So, with the help of local communities and an awareness, necessary steps should be taken to conserve floral diversity. To summarise, the vegetation of coastal sand dunes can be vitally used as therapeutic agents in the medical and pharmaceutical industries, and as such, they must be conserved and further cultivated for the community’s benefit.
Credit author statement
Yuvaraj: Conceptualization, Methodology, Vigneshwar and Kowsalya: Writing- Original draft preparation, Sarah and Praveen: Review and revisions. All the authors approved the manuscript.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors thank the managements of Vel Tech High Tech Dr. Rangarajan Dr. Sakunthala Engineering College, Chennai, Tamil Nadu, India, The Brandenburg University of Technology Cottbus–Senftenberg, Senftenberg, Brandenburg, Germany, Anna University Tiruchirappalli, BIT CAMPUS, Tiruchirappalli, Tamil Nadu, India for support and facilities provided.
- Shrava AK, Patra S, Tikariha A. Uses of weeds as Medicine in Durg District of Chhattisgarh. Indian Journal of Applied and Pure Biology. 2016; 31(1): 91-104.
- Arulmoorthy MP, Srinivasan M. Coastal Sand Dune Floral Diversity in Cuddalore Coastal Areas, Southeast Coast of India MP. Asian Journal of Plant Sciences. 2017; 7(3): 60-64.
- National Institute of Plant Genome research (NIPGR), New Delhi. DBT- National Institute of Plant Genome Research (NIPGR) effort in fight against COVID-19. 2020.
- Fenu G, Cogoni D, Ferrara C, Pinna MS, Bacchetta G. Relationships between coastal sand dune properties and plant community distribution: The case of Is Arenas (Sardinia) Plant. Biosystems. 2012; 146(3): 586–602.
- Elko N, Brodie K, Stockdon H, Nordstrom K, Houser C, McKenna K, Moore Rosati L, Ruggiero P, Thuman R, Walker I. Dune Management Challenges on Developed Coasts. Shore & Beach. 2016; 84(1): 1-14.
- Aparna S, Raja Sekhar PS. Studies on the coastal sand dune phytoresources at Visakhapatnam, Bay of Bengal, India. Asian Journal of Plant Science and Research. 2015; 5(6): 69-76.
- Thirunavukkarasu P, Ramanathan, Ramkumar TL, Balasubramanian T. Anti-Microbial Effect of a Coastal Sand Dune Plant of Spinifex littoreus (Burm f). Merr Current Research Journal of Biological Sciences. 2010; 2(4): 283-285.
- Padmavathy A, Anbarashan M. Phytomedicinal study of coastal sand dune floras in Puducherry. Journal of Medicinal Plants Research. 2011; 5(12): 2566-2571.
- Khan AW, Jan S, Parveen S, Khan RA, Saeed A, Tanveer AJ, Shad AA. Phytochemical analysis and Enzyme Inhibition Assay of Aerva javanica for Ulcer. Chem Cent J. 2012 Jul 31;6(1):76. doi: 10.1186/1752-153X-6-76. PMID: 22849857; PMCID: PMC3477014.
- Bhatia M, Siddiqui N, Gupta S. Abrus Precatorius (L): An Evaluation of Traditional Herb. Indo American Journal of Pharmaceutical Research. 2013; 3:3295-3315.
- Abdul Rahuman A, Gopalakrishnan G, Venkatesan P, Geetha K. Isolation and identification of mosquito larvicidal compound from Abutilon indicum (Linn.) Sweet. Parasitol Res. 2008 Apr;102(5):981-8. doi: 10.1007/s00436-007-0864-5. Epub 2008 Jan 3. PMID: 18176816.
- Arbab AH, Parvez MK, Al-Dosari MS, Al-Rehaily AJ, Al-Sohaibani M, Zaroug EE, AlSaid MS, Rafatullah S. Hepatoprotective and antiviral efficacy of Acacia mellifera leaves fractions against hepatitis B virus. Biomed Res Int. 2015;2015:929131. doi: 10.1155/2015/929131. Epub 2015 Apr 9. PMID: 25950002; PMCID: PMC4407411.
- Chekuri S, Lingfa L, Panjala S, Bindu KCS, Anupalli RR. Acalypha indica L- An important medicinal plant: a brief review of its pharmacological properties and restorative potential. European Journal of Medicinal Plants. 2020; 31(11): 1-10.
- Chiranjibi P, Reddy CS, Dhal NK. Phytomedicinal study of coastal sand dunes species of Orissa, Indian Journal of Traditional Knowledge. 2008; 7(2): 263-268.
- Mukherjee H, Ojha D, Bag P, Chandel HS, Bhattacharyya S, Chatterjee TK, Mukherjee PK, Chakraborti S, Chattopadhyay D. Anti-herpes virus activities of Achyranthes aspera: an indian ethnomedicine, and its triterpene acid. Microbiol Res. 2013 May 6;168(4):238-44. doi: 10.1016/j.micres.2012.11.002. Epub 2012 Dec 5. PMID: 23218996.
- Saleh KA, Albinhassan TH, Elbehairi SEI, Alshehry MA, Alfaifi MY, Al-Ghazzawi AM, Al-Kahtani MA, Alasmari ADA. Cell Cycle Arrest in Different Cancer Cell Lines (Liver, Breast, and Colon) Induces Apoptosis under the Influence of the Chemical Content of Aeluropus lagopoides Leaf Extracts. Molecules. 2019 Jan 31;24(3):507. doi: 10.3390/molecules24030507. PMID: 30708938; PMCID: PMC6384719.
- Al-Omar MS, Mohammed HA, Mohammed SAA, Abd-Elmoniem E, Kandil YI, Eldeeb HM, Chigurupati S, Sulaiman GM, Al-Khurayyif HK, Almansour BS, Suryavamshi PM, Khan RA. Anti-Microbial, Anti-Oxidant, and α-Amylase Inhibitory Activity of Traditionally-Used Medicinal Herbs: A Comparative Analyses of Pharmacology, and Phytoconstituents of Regional Halophytic Plants' Diaspora. Molecules. 2020 Nov 20;25(22):5457. doi: 10.3390/molecules25225457. PMID: 33233786; PMCID: PMC7699972.
- Giday M, Teklehaymanot T. Ethnobotanical study of plants used in management of livestock health problems by Afar people of Ada'ar District, Afar Regional State, Ethiopia. J Ethnobiol Ethnomed. 2013 Jan 23;9:8. doi: 10.1186/1746-4269-9-8. PMID: 23343251; PMCID: PMC3561197.
- Sheela D, Uthayakumari F. GC-MS analysis of bioactive constituents from coastal sand dune taxon – Sesuvium portulacastrum (L), Bioscience Discovery. 2013; 4(1): 47-53.
- Prathibha Bharathi M, Muralidhara Rao D. Evaluation of in vitro antioxidant activity of Aeschynomene indica. Journal of Pharmacy Research. 2015; 9(1):21-26.
- Sahu P, Giri D, Singh R, Pandey P, Gupta S, Shrivastava A, Kumar A, Pandey K.Therapeutic and Medicinal Uses of Aloe vera: A Review. Journal of Pharmacy and Pharmacology. 2013; 4(8):599-610.
- Shehzad A, Qayyum A, Rehman R, Nadeem F, Shehzad M. A Review of Bioactivity Guided Medicinal Uses and Therapeutic Potentials of Noxious Weed (Alternanthera sessilis). International Journal of Chemical and Biochemical Sciences. 2018; 14: 95-103.
- Sridhar KR, Arun AB. Coastal sand dune vegetation and microbial resources: benefits, threats and safeguards. In book: Potential Microorganisms for Sustainable Agriculture – A Techno-Commercial Perspective (Ed.Publisher: IK International Publishing House Pvt. Ltd., New Delhi, IndiaEditors: D.K. Maheshwari, R.C. Dubey 2008; 461-475.
- Lakshmi Narayana V, Narasimharao GM. Plants used in Ethnoveterinary Medicine by Tribals of Visakhapatnam and Vizianagarm Districts, Andhra Pradesh, India. Indian Journal of Pure & Applied Biosciences. 2015; 3(2): 432-439.
- Kumar RP, Sujatha D, Saleem TsM, Chetty CM, Ranganayakulu D. Potential antidiabetic and antioxidant activities of Morus indica and Asystasia gangetica in alloxan-induced diabetes mellitus. J Exp Pharmacol. 2010 Feb 9;2:29-36. doi: 10.2147/jep.s8947. PMID: 27186088; PMCID: PMC4863283.
- Ashfaq UA, Jalil A, Ul Qamar MT. Antiviral phytochemicals identification from Azadirachta indica leaves against HCV NS3 protease: an in silico approach. Nat Prod Res. 2016 Aug;30(16):1866-9. doi: 10.1080/14786419.2015.1075527. Epub 2015 Aug 14. PMID: 26274064.
- Gopalakrishnan S, Kuppuswamy R, Nagaiya R, Arumugam S, Venkatesan G, Ganesan VS. Preliminary Screening of antibacterial compounds from Palar River basin flora. Journal of Phytology. 2010; 2(2): 24–29.
- Nayak S, Behera S, Dash PK. Potential of Microbial Diversity of Coastal Sand Dunes: Need for Exploration in Odisha Coast of India. ScientificWorldJournal. 2019 Jul 14;2019:2758501. doi: 10.1155/2019/2758501. PMID: 31391794; PMCID: PMC6662503.
- Vimalanathan S, Ignacimuthu S, Hudson JB. Medicinal plants of Tamil Nadu (Southern India) are a rich source of antiviral activities. Pharmaceutical Biology. 2009; 47(5): 422–429.
- Kiran DP, Amit VY, Yogesh SS, Shubhada RT. Influence of endophytic fungal elicitation on production of inophyllum in suspension cultures of Calophyllum inophyllum L, Plant Cell Tissue and Organ Culture. 2011; 106: 345–352.
- Verma HN, Awasthi LP. Antiviral activity of Boerhaavia diffusa root extract and the physical properties of the virus inhibitor. Canadian Journal of Botany. 2012; 57(8): 926-932.
- Ono M, Takigawa A, Muto H, Kabata K, Okawa M, Kinjo J, Yokomizo K, Yoshimitsu H, Nohara T. Antiviral Activity of Four New Resin Glycosides Calysolins XIV-XVII from Calystegia soldanella against Herpes Simplex Virus. Chem Pharm Bull (Tokyo). 2015;63(8):641-8. doi: 10.1248/cpb.c15-00307. PMID: 26235171.
- Lee JI, Kim IH, Nam TJ. Crude extract and solvent fractions of Calystegia soldanella induce G1 and S phase arrest of the cell cycle in HepG2 cells. Int J Oncol. 2017 Feb;50(2):414-420. doi: 10.3892/ijo.2017.3836. Epub 2017 Jan 2. PMID: 28101580; PMCID: PMC5238786.
- Vedavayas R, Niveditha K, Sridhar R. Concanavalin and Canavanine in seeds of Coastal sand dune legumes (Canavalia). Advanced Biotech. 2012; 11(10): 30-33.
- Venkanna L, Estari. Inhibition of Human Immunodeficiency Virus (HIV-1) Reverse Transcriptase by Casia occidentalis (L), Plant Extract. International Journal of Scientific and Engineering Research. 2012; 3(7).
- Al-Snafi A. The therapeutic importance of Cassia occidentalis-an overview Indian Journal of Pharmaceutical Sciences. 2015; 5(3): 158-171.
- Balasubramanian P, Jayalakshmi K, Vidhya N, Prasad R, Sheriff AK, Kathiravan G, Rajagopal K, Sureban SM. Antiviral activity of ancient system of ayurvedic medicinal plant Cissus quadrangularis L. (Vitaceae). J Basic Clin Pharm. 2009 Dec;1(1):37-40. Epub 2010 Feb 15. PMID: 25206252; PMCID: PMC4158892.
- Chinelo AE, Sandra NN. Comparison of phytochemical and proximate compositions of parts of Cleome ciliata Schum & Thonn and Cleome viscosa L. International Journal Of Pharmaceutical and Bio-Medical Science. 2015; 1: 1-5199.
- Lima EB, Sousa CN, Meneses LN, Ximenes NC, Santos Júnior MA, Vasconcelos GS, Lima NB, Patrocínio MC, Macedo D, Vasconcelos SM. Cocos nucifera (L.) (Arecaceae): A phytochemical and pharmacological review. Braz J Med Biol Res. 2015 Nov;48(11):953-64. doi: 10.1590/1414-431X20154773. Epub 2015 Aug 18. PMID: 26292222; PMCID: PMC4671521.
- Maregesi SM, Pieters L, Ngassapa OD, Apers S, Vingerhoets R, Cos P, Berghe DA, Vlietinck AJ. Screening of some Tanzanian medicinal plants from Bunda district for antibacterial, antifungal and antiviral activities. J Ethnopharmacol. 2008 Sep 2;119(1):58-66. doi: 10.1016/j.jep.2008.05.033. Epub 2008 Jun 6. PMID: 18582554.
- Wilian de Oliveir R, Evandro Jose LR, Paula Carvalhal L, Von Buettner R, Eudes da Silva V, Diego de Carvalho C, Bruno Suzana LB, Telles da Cunha L. Isolation, characterization and analysis of the agglutinative activity of a lectin from Crotalaria spectabilis. Journal of Plant Biochemistry and Biotechnology. 2018; 27: 373–381.
- Tasnova N. Investigation of in-vitro Cytotoxic and Antibacterial Activity of Methanol Extract of Crotalaria verrucosa Leaves, Institutional Repository, Department of Pharmacy, BRAC University, Bangladesh. 2016; 1-56.
- Dingse P, Marina S, Farha D, Febby K. Diversity of medicinal plants and their uses by the Sanger tribe of Sangihe Islands, North Sulawesi, Indonesia. Journal of Biological Diversity. 2019; 20(2): 611-621.
- Murali KS, Sivasubramanian S, Vincent S, Murugan SB, Giridaran B, Dinesh S, Gunasekaran P, Krishnasamy K, Sathishkumar R. Anti-chikungunya activity of luteolin and apigenin rich fraction from Cynodon dactylon. Asian Pac J Trop Med. 2015 May;8(5):352-8. doi: 10.1016/S1995-7645(14)60343-6. PMID: 26003593.
- Soltan MM, Zaki AK. Antiviral screening of forty-two Egyptian medicinal plants. J Ethnopharmacol. 2009 Oct 29;126(1):102-7. doi: 10.1016/j.jep.2009.08.001. Epub 2009 Aug 8. PMID: 19666102.
- Ali Esmail A. Iraqi Medicinal Plants with Antiviral Effect- A Review. IOSR Journal of Pharmacy. 2019; 9(7): 57-75.
- Maikaeo L, Chotigeat W, Mahabusarakam W. Emilia sonchifolia extract activity against white spot syndrome virus and yellow head virus in shrimp cell cultures. Dis Aquat Organ. 2015 Jul 23;115(2):157-64. doi: 10.3354/dao02891. PMID: 26203887.
- Rasool N, Aisha A, Muneeba W, Waqar H, Sajid M. Computational exploration of antiviral activity of phytochemicals against NS2B/NS3 proteases from dengue virus. Turkish Journal of Biochemistry. 2019; 44(3): 261-277.
- Ghosh M, Civra A, Rittà M, Cagno V, Mavuduru SG, Awasthi P, Lembo D, Donalisio M. Ficus religiosa L. bark extracts inhibit infection by herpes simplex virus type 2 in vitro. Arch Virol. 2016 Dec;161(12):3509-3514. doi: 10.1007/s00705-016-3032-3. Epub 2016 Aug 31. PMID: 27581805.
- Marius H, Kazhila Chinsembu C. Ethnomedicinal study of plants used to manage HIV/AIDS-related disease conditions in the Ohangwena region, Namibia. International Journal of Medicinal Plants Research. 2012; 1(1): 4-11.
- Ain Satish C, Pancholi B, Singh R, Jain R. Pharmacognostical studies of important arid zone plants. Revista Brasileira de Farmacognosia. 2010; 20(5): 659-665.
- Consolacion YR, Dinah LE, Emelina HM, Ming-Jaw D, Chien-Chang S. A new triterpene from Glinus oppositifolius, Chinese Journal of Natural Medicines. 2012; 10(4): 0284-0286.
- Krishnan SRS, Siril EA. Elicitor and precursor mediated anthraquinone production from cell suspension cultures of Oldenlandia umbellate. IJPSR. 2016; 7(9): 3649-3657.
- Li DL, Li XM, Wang BG. Natural anthraquinone derivatives from a marine mangrove plant-derived endophytic fungus Eurotium rubrum: structural elucidation and DPPH radical scavenging activity. J Microbiol Biotechnol. 2009 Jul;19(7):675-80. PMID: 19652514.
- Sangeetha K, Rajarajan S. Evaluation of in vitro antiviral activity of Vitex negundo L, Hyptis suaveolens (L) poit, Decalepis hamiltonii Wight & Arn, to Chikungunya virus. Asian Pacific Journal of Tropical Disease. 2014; 4(1):111-115.
- Danmalam UH, Abdullahi LM, Agunu A, Musa KY. Acute toxicity studies and hypoglycemic activity of the methanol extract of the leaves of Hyptis suaveolens Poit (Lamiaceae). Nigerian Journal of Pharmaceutical Research. 2019; 8(2): 87-92.
- Frey FM, Meyers R. Antibacterial activity of traditional medicinal plants used by Haudenosaunee peoples of New York State. BMC Complement Altern Med. 2010 Nov 6;10:64. doi: 10.1186/1472-6882-10-64. PMID: 21054887; PMCID: PMC2989932.
- Meira S, Marilena D, Eliezer Pereira M, David Jorge P, Juceni D. Review of the genus Ipomoea: traditional uses, chemistry and biological activities, Rev bras Farmacogn. 2012; 22(3): 682-713.
- Ramesh A, Sundarraj P, Balamani J. Phytochemical evaluation of leaf and stem of Ipomoea pes-caprae (L)R.Br. International Journal of Advanced Research. 2019; 7(1):139-149.
- Md Mizanur Rahman M, Anik B, Saifuddin Mohammad S, Monirul Islam ASM, Mahadi Hassan B. Phytochemical Screening, Cytotoxic and Anthelmintic activities of Amorphophallus campanulatus (Roxb), Avicennia marina (Forssk) and Launaea sarmentosa (Willd). Bangladesh Journal of Pharmacology. 2016; 19(1): 106-113.
- Tsai YC, Hohmann J, El-Shazly M, Chang LK, Dankó B, Kúsz N, Hsieh CT, Hunyadi A, Chang FR. Bioactive constituents of Lindernia crustacea and its anti-EBV effect via Rta expression inhibition in the viral lytic cycle. J Ethnopharmacol. 2020 Mar 25;250:112493. doi: 10.1016/j.jep.2019.112493. Epub 2019 Dec 18. PMID: 31863859.
- Lampariello LR, Cortelazzo A, Guerranti R, Sticozzi C, Valacchi G. The Magic Velvet Bean of Mucuna pruriens. J Tradit Complement Med. 2012 Oct;2(4):331-9. doi: 10.1016/s2225-4110(16)30119-5. PMID: 24716148; PMCID: PMC3942911.
- Siva R, Rajasekaran C, Mudgal G. Induction of somatic embryogenesis and organogenesis in Oldenlandia umbellata L, a dye-yielding medicinal plant. Plant Cell, Tissue and Organ Culture. 1998; 20: 205–211.
- Kunyanga CN, Vellingiri V, Imungi KJ. Nutritional quality, phytochemical composition and health protective effects of an under-utilized prickly cactus fruit (Opuntia stricta Haw) collected from Kenya. African Journal of Food, Agriculture, Nutrition and Development. 2014; 14(7).
- Adkar PP, Bhaskar VH. Pandanus odoratissimus (Kewda): A Review on Ethnopharmacology, Phytochemistry, and Nutritional Aspects. Adv Pharmacol Sci. 2014;2014:120895. doi: 10.1155/2014/120895. Epub 2014 Dec 22. PMID: 25949238; PMCID: PMC4408760.
- Ishwarya R, Vaseeharan B, Anuradha R, Rekha R, Govindarajan M, Alharbi NS, Kadaikunnan S, Khaled JM, Benelli G. Eco-friendly fabrication of Ag nanostructures using the seed extract of Pedalium murex, an ancient Indian medicinal plant: Histopathological effects on the Zika virus vector Aedes aegypti and inhibition of biofilm-forming pathogenic bacteria. J Photochem Photobiol B. 2017 Sep;174:133-143. doi: 10.1016/j.jphotobiol.2017.07.026. Epub 2017 Jul 25. PMID: 28772238.
- Abdelhakim B, Youssef B, El Ouardy K, Fatima E, Ahmed T, Abdeslam T, Et Jamal A, Nadia D. Antibacterial, antioxidant and antitumor properties of Moroccan medicinal plants: A review. Asian Pacific Journal of Tropical Disease. 2017; 7(1): 57-64.
- Mohamed RE, Abdel Nasser S, Eman El-Taher MM, Mona Kassem ES. A Comprehensive Review of Phoenix (Arecaceae). Research Journal of Pharmaceutical, Biological and Chemical Sciences. 2015; 6(3):966.
- Zainul A, Muhammad Q, Aysha R, Yousuf Adnan M, Bilquess G, Ajmal Khan M. Antioxidant Activity and Polyphenolic Content of Phragmites Karka Under Saline Conditions. Pakistan Journal of Botany. 2015; 47(3): 813-818.
- de Brito Damasceno GA, Souto AL, da Silva IB, Roque A, Ferrari M, Giordani RB. Prosopis juliflora: Phytochemical, Toxicological, and Allelochemicals In: Merillon JM, Ramawat K (eds) Co-Evolution of Secondary Metabolites, Reference Series in Phytochemistry, Springer. 8(9):187-191.
- Sharma RA, Renu SA. Review on Phyla nodiflora Linn: A Wild Wetland Medicinal Herb. International Journal of Pharmaceutical Sciences Review and Research. 2013; 20(1):57-63.
- Farid MM, Marzouk MM, Hussein SR, Elkhateeb A, Abdel-Hameed ES. Comparative study of Posidonia oceanica L: LC/ESI/MS analysis, cytotoxic activity and chemosystematic significance. Journal of Materials and Environmental Science. 2018; 9(6): 1676-1682.
- Marwat SK, Rehman F, Khan EA, Baloch MS, Sadiq M, Ullah I, Javaria S, Shaheen S. Review - Ricinus cmmunis - Ethnomedicinal uses and pharmacological activities. Pak J Pharm Sci. 2017 Sep;30(5):1815-1827. PMID: 29084706.
- Patel S. Salicornia: evaluating the halophytic extremophile as a food and a pharmaceutical candidate. 3 Biotech. 2016 Jun;6(1):104. doi: 10.1007/s13205-016-0418-6. Epub 2016 Apr 18. PMID: 28330174; PMCID: PMC4835422.
- Ahmad H, Rajagopal K. Biological Activities of Salvadora persica L (Meswak). Medicinal and Aromatic Plants. 2013; 2(4):129.
- Goswami S, Mishra K, Singh R, Singh P, Pradeep S. Sesbania- A Plant with Diverse Therapeutic Benefits: An Overview. Indian Journal of Pharmaceutical Education and Research. 2016; 1: 1-13.
- Manbir Nitika K. Review on Sea purslane. Journal of Pharmacognosy and Phytochemistry. 2015; 3(5):22-24.
- Galal A, Raman V, Khan Ikhlas A. Sida cordifolia, a Traditional Herb in Modern Perspective – A Review. Current Traditional Medicine. 2015; 11(13):5-17.
- Kokila Parmar A, Anup Patel N. Preliminary Phytochemical Screening and study of antiviral activity and antibacterial activity of Tephrosia purpurea flower, Life Sciences Leaflets. 2010; 1: 7-13.
- Arunkumar J, Rajarajan S. A Study on the in vitro Cytotoxicity and Anti- HSV-2 Activity of Lyophilized Extracts of Terminalia Catappa Lin, Mangifera Indica Lin and Phytochemical Compound Mangiferin. Journal of Medical Virology. 2015; 2: 22-26.
- Saravanakumar A, Renukadevi P, Vanitha J, Venkateshwaran K, Ganesh M, De Clercq E. Evaluation of Antiviral and Cytotoxic Activities of Methanolic Extract of Thespesia Populnea (Malvaceae) Flowers. Journal of Herbs, Spices & Medicinal Plants. 2011; 17(4):386-391.
- Anuya Aparna AR, Abhay Sadashiv C. Evaluation of Ocimum sanctum and Tinospora cordifolia as Probable HIV-Protease Inhibitors. International Journal of Pharmaceutical Sciences Review and Research. 2014; 25(1):315-318.
- Saranya N, Jayakanthan M, Caroline R, Kandavelmani A, Bharathi N, Kumaravadivel N, Gnanam R, Raveendran M, Mohankumar S, Kumar N. Shortlisting Phytochemicals Exhibiting Inhibitory Activity against Major Proteins of SARS-CoV-2 through Virtual Screening. Frontiers in Pharmacology. 2020; 1-26.
- Lonard RI, Judd FW, Stalter R. Biological Flora of Coastal Dunes and Wetlands: Uniola paniculata L. Journal of Coastal Research. 2011; 276: 984–993.
- Akassh M, Fathima T, Mruthunjaya K. Health Promoting Effects of Ziziphus mauritiana: An Overview. International Journal of Pharmaceutical Sciences and Research. 2020; 11(1).
- Riffat B, Ejaz A, Tariq M, Benny Tan KH, Vincent Chow TK. Inhibitory activities of extracts of Rumex dentatus, Commelina benghalensis, Ajuga bracteosa, Ziziphus mauritiana as well as their compounds of gallic acid and emodin against dengue virus. Asian Pacific Journal of Tropical Medicine. 2018; 11(4): 265-271.
- Rupa R, Ekta S, Goyal SK. Antimicrobial activity of Zoysia grass (Turf Grass/Lawn Grass) on Total Coliform: A low-cost potential Water disinfectants International Journal of Advanced Research. 2016; 4(1): 239- 248.
- Savoia D. Plant-derived antimicrobial compounds: alternatives to antibiotics. Future Microbiol. 2012 Aug;7(8):979-90. doi: 10.2217/fmb.12.68. PMID: 22913356.
- Vaou N, Stavropoulou E, Voidarou C, Tsigalou C, Bezirtzoglou E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms. 2021 Sep 27;9(10):2041. doi: 10.3390/microorganisms9102041. PMID: 34683362; PMCID: PMC8541629.
- Anup KC, Amit VG, Karuna BS. Phytopharmacological review on Acanthospermum Hispidum. Journal of Applied Pharmaceutical Science. 2012; 2(1): 144-148.
- Anup KC, Amit VG, Karuna BS. Phytopharmacological review on Acanthospermum Hispidum. Journal of Applied Pharmaceutical Science. 2012; 2(1): 144-148.
- Dutta S, Dey P, Chaudhuri TK. Quantification and Correlation of the Bioactive Phytochemicals of Croton Bonplandianum Leaves of Sub-Himalayan Region of West Bengal. Asian Journal of Pharmaceutical and Clinical Research. 2013; 6:3.
- Iswaryalakshmi K, Akilabalamurugan M. Phytochemical screening antioxidant and antimicrobial activity of Aeschynomene aspera linn root extract. International Jourdnal of Pharmacy and Biological Sciences. 2019; 7(1): 29-33.
- Khan FM. Ethno-veterinary medicinal usage of flora of greater Cholistan desert, Pakistan. Pakistan Veterinary Journal. 2009; 29(2): 75-80.
- Kumar Sharma A. Medicinal properties of bala (Sida Cordifolia Linn and its species). International Journal of Ayurveda and Pharma Research. 2015; 1(2):1-9.
- Lim TK. Vigna unguiculata cv-gr Unguiculata In: Edible Medicinal and Non-Medicinal Plants Springer, Dordrecht, 2012; 971-975.
- Motamarri NS, Karthikeyan M, Rajasekar S, Gopal V. Indigofera tinctoria Linn - a phytopharmacological review. International Journal of Pharmaceutical and Bio-Medical Science. 2012; 3(1): 164-169.
- Nouran Fahmy M, Al-Sayed E, Saad M, Faizul A, Mohamed El-Shazly Abdel Nasser S. Breaking down the barriers to a natural antiviral agent: Antiviral activity and molecular docking of Erythrina speciosa extract, fractions and the major compound Chemistry & Biodiversity. 2019; 5(11).
- Rajeshwari S, Sevarkodiyone SP. Medicinal properties of Abutilon Indicum, Open Journal of Plant Science. 2018; 3(1): 22-25.
- Rangra N, Samanta S, Pradhan K. A comprehensive review on phytopharmacological investigations of Acacia auriculiformis A.Cunn. ex Benth. Asian Pacific Journal of Tropical Biomedicine. 2019; 9(1): 1-11.
- Ratnasooriya WD, Pathirana RN, Dissanayake AS, Samanmali BLC, Desman PK. Evaluation of invitro sun screen activities of salt marshy plants Suaeda monoica, Suaeda maritima and Halosarcia indica, International Journal of Pharmaceutical Research and Allied Sciences. 2016; 5(2):15-20.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
